Clinical Significance and the Role of Guanylate-Binding Protein 5 in Oral Squamous Cell Carcinoma
- PMID: 34439200
- PMCID: PMC8394330
- DOI: 10.3390/cancers13164043
Clinical Significance and the Role of Guanylate-Binding Protein 5 in Oral Squamous Cell Carcinoma
Abstract
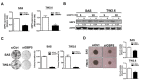
Guanylate binding protein 5 (GBP5) is the interferon (IFN)-inducible subfamily of guanosine triphosphatases (GTPases) and is involved in pathogen defense. However, the role played by GBP5 in cancer development, especially in oral squamous cell carcinoma (OSCC), is still unknown. Herein, next-generation sequencing analysis showed that the gene expression levels of GBP5 were significantly higher in OSCC tissues compared with those found in corresponding tumor adjacent normal tissues (CTAN) from two pairs of OSCC patients. Higher gene expression levels of GBP5 were also found in tumor tissues of 23 buccal mucosal squamous cell carcinoma (BMSCC)/14 tongue squamous cell carcinoma (TSCC) patients and 30 oral cancer patients from The Cancer Genome Atlas (TCGA) database compared with those in CTAN tissues. Immunohistochemical results showed that protein expression levels of GBP5 were also higher in the tumor tissues of 353 OSCC patients including 117 BMSCC, 187 TSCC, and 49 lip squamous cell carcinoma patients. Moreover, TCGA database analysis indicated that high gene expression levels of GBP5 were associated with poor overall survival in oral cancer patients with moderate/poor cell differentiation, and associated with poor disease-free survival in oral cancer patients with moderate/poor cell differentiation and lymph node metastasis. Furthermore, GBP5-knockdowned cells exhibited decreased cell growth, arrest at G1 phase, and decreased invasion/migration. The gene expression of markers for epithelial-mesenchymal transition and cancer stemness was also reduced in GBP5-silenced oral cancer cells. Taken together, GBP5 might be a potential biomarker and therapeutic target for OSCC patients, especially for those with poor cell differentiation and lymph node metastasis.
Keywords: guanylate binding protein 5; malignancy; oral squamous cell carcinoma; prognosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

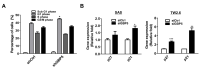


References
-
- Spoerl S., Gerken M., Mamilos A., Fischer R., Wolf S., Nieberle F., Klingelhoffer C., Meier J.K., Spoerl S., Ettl T., et al. Lymph node ratio as a predictor for outcome in oral squamous cell carcinoma: A multicenter population-based cohort study. Clin. Oral Investig. 2021;25:1705–1713. doi: 10.1007/s00784-020-03471-6. - DOI - PMC - PubMed
-
- Vestal D.J., Jeyaratnam J.A. The guanylate-binding proteins: Emerging insights into the biochemical properties and functions of this family of large interferon-induced guanosine triphosphatase. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2011;31:89–97. doi: 10.1089/jir.2010.0102. - DOI - PMC - PubMed
Grants and funding
- MOST 108-2320-B-037-038 and MOST 109-2320-B-037-015-MY3/The Ministry of Science and Technology
- ZBH 107-19/Kaohsiung Armed Forces General Hospital
- KMU-Q109008 andKMU-Q110002/Kaohsiung Medical University Research Foundation
- NSYSUKMU 109-I007 and NSYSUKMU-110-I006/The National Sun Yat-sen University-KMU Joint Research Project
- VGHKS106-152 and VGHKS106-158/Kaohsiung Veterans General Hospital
LinkOut - more resources
Full Text Sources

