Molecularly Targeted Therapies for Gastric Cancer. State of the Art
- PMID: 34439248
- PMCID: PMC8392056
- DOI: 10.3390/cancers13164094
Molecularly Targeted Therapies for Gastric Cancer. State of the Art
Abstract
Many phase III trials failed to demonstrate a survival benefit from the addition of molecular therapy to conventional chemotherapy for advanced and metastatic gastric cancer, and only three agents were approved by the FDA. We examined the efficacy and safety of novel drugs recently investigated. PubMed, Embase and Cochrane Library were searched for phase III randomized controlled trials published from January 2016 to December 2020. Patients in the experimental arm received molecular therapy with or without conventional chemotherapy, while those in the control arm had conventional chemotherapy alone. The primary outcomes were overall and progression-free survival. The secondary outcomes were the rate of tumor response, severe adverse effects, and quality of life. Eight studies with a total of 4223 enrolled patients were included. The overall and progression-free survival of molecular and conventional therapy were comparable. Most of these trials did not find a significant difference in tumor response rate and in the number of severe adverse effects and related deaths between the experimental and control arms. The survival benefits of molecular therapies available to date for advanced and metastatic gastric cancer are rather unclear, mostly due to inaccurate patient selection, particularly concerning oncogene amplification and copy number.
Keywords: EGFR inhibitors; MET inhibitors; angiogenesis inhibitors; chemotherapy; gastric cancer; molecular target therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

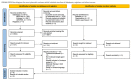




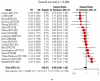

References
-
- Hartgrink H., Van De Velde C., Putter H., Bonenkamp J., Kranenbarg E.M.-K., Songun V., Welvaart K., Van Krieken J., Meijer S., Plukker J., et al. Extended Lymph Node Dissection for Gastric Cancer: Who May Benefit? Final Results of the Randomized Dutch Gastric Cancer Group Trial. J. Clin. Oncol. 2004;22:2069–2077. doi: 10.1200/JCO.2004.08.026. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

