Smart Modification on Magnetic Nanoparticles Dramatically Enhances Their Therapeutic Properties
- PMID: 34439250
- PMCID: PMC8391586
- DOI: 10.3390/cancers13164095
Smart Modification on Magnetic Nanoparticles Dramatically Enhances Their Therapeutic Properties
Abstract
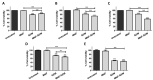
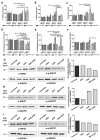
Magnetic nanoparticles (MNP) are employed as nanocarriers and in magnetic hyperthermia (MH) for the treatment of cancers. Herein, a smart drug delivery system composed of MNP functionalized with the cytotoxic drug gemcitabine (MNP-GEM) has been thoroughly evaluated. The linker employed is based on a disulfide bond and allows the controlled release of GEM under a highly reducing environment, which is frequently present in the cytoplasm of tumor cells. The stability, MH, and the interaction with plasma proteins of the nanoparticles are evaluated, highlighting their great potential for biological applications. Their cytotoxicity is assessed in three pancreatic cancer cell lines with different sensitivity to GEM, including the generation of reactive oxygen species (ROS), the effects on the cell cycle, and the mechanisms of cell death involved. Remarkably, the proposed nanocarrier is better internalized than unmodified nanoparticles, and it is particularly effective in PANC-1 cells, resistant to GEM, but not in non-tumoral keratinocytes. Additionally, its combination with MH produces a synergistic cytotoxic effect in all cancer cell lines tested. In conclusion, MNP-GEM presents a promising potential for treating pancreatic cancer, due to multiple parameters, such as reduced binding to plasma proteins, increased internalization, and synergistic activity when combined with MH.
Keywords: drug delivery; magnetic hyperthermia; magnetic nanoparticles; nanomedicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

