Experimental and Clinical Evidence Supports the Use of Urokinase Plasminogen Activation System Components as Clinically Relevant Biomarkers in Gastroesophageal Adenocarcinoma
- PMID: 34439251
- PMCID: PMC8393967
- DOI: 10.3390/cancers13164097
Experimental and Clinical Evidence Supports the Use of Urokinase Plasminogen Activation System Components as Clinically Relevant Biomarkers in Gastroesophageal Adenocarcinoma
Abstract
Gastric and oesophageal cancers (GOCs) are lethal cancers which metastasise early and recur frequently, even after definitive surgery. The urokinase plasminogen activator system (uPAS) is strongly implicated in the invasion and metastasis of many aggressive tumours including GOCs. Urokinase plasminogen activator (uPA) interaction with its receptor, urokinase plasminogen activator receptor (uPAR), leads to proteolytic activation of plasminogen to plasmin, a broad-spectrum protease which enables tumour cell invasion and dissemination to distant sites. uPA, uPAR and the plasminogen activator inhibitor type 1 (PAI-1) are overexpressed in some GOCs. Accumulating evidence points to a causal role of activated receptor tyrosine kinase pathways enhancing uPAS expression in GOCs. Expression of these components are associated with poorer clinicopathological features and patient survival. Stromal cells, including tumour-associated macrophages and myofibroblasts, also express the key uPAS proteins, supporting the argument of stromal involvement in GOC progression and adverse effect on patient survival. uPAS proteins can be detected on circulating leucocytes, circulating tumour cells and within the serum; all have the potential to be developed into circulating biomarkers of GOC. Herein, we review the experimental and clinical evidence supporting uPAS expression as clinical biomarker in GOC, with the goal of developing targeted therapeutics against the uPAS.
Keywords: biomarkers; circulating tumour cell (CTC); gastric cancer; oesophageal cancer; plasminogen activator inhibitor type 1 (PAI-1); serine proteases; serpins; tumour microenvironment; urokinase plasminogen activator (uPA); urokinase plasminogen activator receptor (uPAR).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


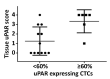
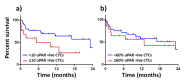
References
-
- Wilkinson N., Howe J., Gay G., Patel-Parekh L., Scott-Conner C., Donohue J. Differences in the pattern of presentation and treatment of proximal and distal gastric cancer: Results of the 2001 gastric patient care evaluation. Ann. Surg. Oncol. 2008;15:1644–1650. doi: 10.1245/s10434-008-9877-2. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

