Efficacy of Fulvestrant in Women with Hormone-Resistant Metastatic Breast Cancer (mBC): A Canadian Province Experience
- PMID: 34439317
- PMCID: PMC8391338
- DOI: 10.3390/cancers13164163
Efficacy of Fulvestrant in Women with Hormone-Resistant Metastatic Breast Cancer (mBC): A Canadian Province Experience
Abstract
Introduction: Fulvestrant has demonstrated efficacy in hormone receptor positive (HR+) metastatic breast cancer (mBC), both in first-and second-line settings. In clinical practice, however, fulvestrant has been used as a later-line therapy. This study assessed the efficacy of fulvestrant in women with mBC in early-versus later-line therapy.
Methods: This retrospective cohort study assessed Saskatchewan women with HR+ mBC who received fulvestrant between 2003-2019. A multivariate Cox proportional survival analysis was performed.
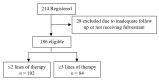
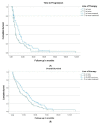
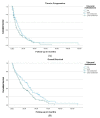
Results: One hundred and eighty-six women with a median age of 63.5 years were identified-178 (95.6%) had hormone-resistant mBC, 57.5% had visceral disease, and 43.0% had received chemotherapy before fulvestrant. 102 (54.8%) women received ≤2-line-therapy, and 84 (45.2%) received ≥3 line-therapy before fulvestrant. The median time to progression (TTP) was 12 months in the early-treatment vs. 6 months in the later-treatment group, p = 0.015. Overall survival (OS) from the start of fulvestrant was 26 months in the early-treatment group vs. 16 months in the later-treatment group, p = 0.067. On multivariate analysis, absence of visceral metastasis, HR: 0.70 (0.50-0.99), was significantly correlated with better TTP, whereas post-fulvestrant chemotherapy, HR: 0.32 (0.23-0.47), clinical benefit from fulvestrant, HR: 0.44 (0.30-0.65), and absence of visceral metastasis, HR: 0.70 (0.50-0.97), were correlated with better OS.
Conclusions: Fulvestrant has demonstrated efficacy as both early-and later-line therapy in hormone-resistant mBC. Our results show that women with clinical benefit from fulvestrant, who received post-fulvestrant chemotherapy, or had non-visceral disease, had better survival.
Keywords: fulvestrant; hormone receptor positive breast cancer; hormone-resistant; survival; visceral metastases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Parise C.A., Bauer K.R., Brown M.M., Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J. 2009;15:593. doi: 10.1111/j.1524-4741.2009.00822.x. - DOI - PubMed
-
- Hammond M.E., Hayes D.F., Dowsett M., Allred D.C., Hagerty K.L., Badve S., Fitzgibbons P.L., Francis G., Goldstein N.S., Hayes M., et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010;28:2784. doi: 10.1200/JCO.2009.25.6529. - DOI - PMC - PubMed
-
- Female Breast Cancer Subtypes—Cancer Stat Facts [Internet]. SEER. [(accessed on 28 December 2020)]; Available online: https://seer.cancer.gov/statfacts/html/breast-subtypes.html.
Grants and funding
LinkOut - more resources
Full Text Sources

