Identification of L-Cysteinamide as a Potent Inhibitor of Tyrosinase-Mediated Dopachrome Formation and Eumelanin Synthesis
- PMID: 34439449
- PMCID: PMC8388879
- DOI: 10.3390/antiox10081202
Identification of L-Cysteinamide as a Potent Inhibitor of Tyrosinase-Mediated Dopachrome Formation and Eumelanin Synthesis
Abstract
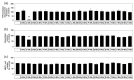
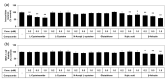
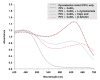
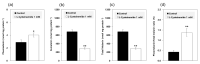
The purpose of this study is to identify amino acid derivatives with potent anti-eumelanogenic activity. First, we compared the effects of twenty different amidated amino acids on tyrosinase (TYR)-mediated dopachrome formation in vitro and melanin content in dark-pigmented human melanoma MNT-1 cells. The results showed that only L-cysteinamide inhibited TYR-mediated dopachrome formation in vitro and reduced the melanin content of cells. Next, the antimelanogenic effect of L-cysteinamide was compared to those of other thiol compounds (L-cysteine, N-acetyl L-cysteine, glutathione, L-cysteine ethyl ester, N-acetyl L-cysteinamide, and cysteamine) and positive controls with known antimelanogenic effects (kojic acid and β-arbutin). The results showed the unique properties of L-cysteinamide, which effectively reduces melanin content without causing cytotoxicity. L-Cysteinamide did not affect the mRNA and protein levels of TYR, tyrosinase-related protein 1, and dopachrome tautomerase in MNT-1 cells. L-Cysteinamide exhibited similar properties in normal human epidermal melanocytes (HEMs). Experiments using mushroom TYR suggest that L-cysteinamide at certain concentrations can inhibit eumelanin synthesis through a dual mechanism by inhibiting TYR-catalyzed dopaquinone synthesis and by diverting the synthesized dopaquinone to the formation of DOPA-cysteinamide conjugates rather than dopachrome. Finally, L-cysteinamide was shown to increase pheomelanin content while decreasing eumelanin and total melanin contents in MNT-1 cells. This study suggests that L-cysteinamide has an optimal structure that can effectively and safely inhibit eumelanin synthesis in MNT-1 cells and HEMs, and will be useful in controlling skin hyperpigmentation.
Keywords: L-cysteinamide; MNT-1 melanoma; eumelanin; melanin; normal human melanocytes; tyrosinase; viability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Slominski A., Kim T.-K., Brozyna A.A., Janjetovic Z., Brooks D.L., Schwab L.P., Skobowiat C., Jóźwicki W., Seagroves T.N. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways. Arch. Biochem. Biophys. 2014;563:79–93. doi: 10.1016/j.abb.2014.06.030. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

