Garcinia cambogia Ameliorates Non-Alcoholic Fatty Liver Disease by Inhibiting Oxidative Stress-Mediated Steatosis and Apoptosis through NRF2-ARE Activation
- PMID: 34439474
- PMCID: PMC8388869
- DOI: 10.3390/antiox10081226
Garcinia cambogia Ameliorates Non-Alcoholic Fatty Liver Disease by Inhibiting Oxidative Stress-Mediated Steatosis and Apoptosis through NRF2-ARE Activation
Abstract
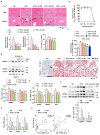
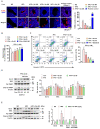
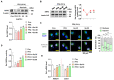
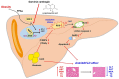
Excessive free fatty acids (FFAs) causes reactive oxygen species (ROS) generation and non-alcoholic fatty liver disease (NAFLD) development. Garcinia cambogia (G. cambogia) is used as an anti-obesity supplement, and its protective potential against NAFLD has been investigated. This study aims to present the therapeutic effects of G. cambogia on NAFLD and reveal underlying mechanisms. High-fat diet (HFD)-fed mice were administered G. cambogia for eight weeks, and steatosis, apoptosis, and biochemical parameters were examined in vivo. FFA-induced HepG2 cells were treated with G. cambogia, and lipid accumulation, apoptosis, ROS level, and signal alterations were examined. The results showed that G. cambogia inhibited HFD-induced steatosis and apoptosis and abrogated abnormalities in serum chemistry. G. cambogia increased in NRF2 nuclear expression and activated antioxidant responsive element (ARE), causing induction of antioxidant gene expression. NRF2 activation inhibited FFA-induced ROS production, which suppressed lipogenic transcription factors, C/EBPα and PPARγ. Moreover, the ability of G. cambogia to inhibit ROS production suppressed apoptosis by normalizing the Bcl-2/BAX ratio and PARP cleavage. Lastly, these therapeutic effects of G. cambogia were due to hydroxycitric acid (HCA). These findings provide new insight into the mechanism by which G. cambogia regulates NAFLD progression.
Keywords: Garcinia cambogia; NRF2; antioxidant; apoptosis; hydroxycitric acid; non-alcoholic fatty liver disease (NAFLD); reactive oxygen species; steatosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

