Fecal Transplant and Bifidobacterium Treatments Modulate Gut Clostridium Bacteria and Rescue Social Impairment and Hippocampal BDNF Expression in a Rodent Model of Autism
- PMID: 34439657
- PMCID: PMC8391663
- DOI: 10.3390/brainsci11081038
Fecal Transplant and Bifidobacterium Treatments Modulate Gut Clostridium Bacteria and Rescue Social Impairment and Hippocampal BDNF Expression in a Rodent Model of Autism
Abstract
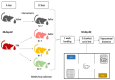
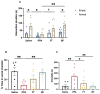
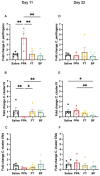
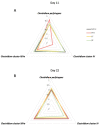
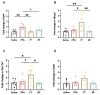
Autism is associated with gastrointestinal dysfunction and gut microbiota dysbiosis, including an overall increase in Clostridium. Modulation of the gut microbiota is suggested to improve autistic symptoms. In this study, we explored the implementation of two different interventions that target the microbiota in a rodent model of autism and their effects on social behavior: the levels of different fecal Clostridium spp., and hippocampal transcript levels. Autism was induced in young Sprague Dawley male rats using oral gavage of propionic acid (PPA) for three days, while controls received saline. PPA-treated animals were divided to receive either saline, fecal transplant from healthy donor rats, or Bifidobacterium for 22 days, while controls continued to receive saline. We found that PPA attenuated social interaction in animals, which was rescued by the two interventions. PPA-treated animals had a significantly increased abundance of fecal C. perfringens with a concomitant decrease in Clostridium cluster IV, and exhibited high hippocampal Bdnf expression compared to controls. Fecal microbiota transplantation or Bifidobacterium treatment restored the balance of fecal Clostridium spp. and normalized the level of Bdnf expression. These findings highlight the involvement of the gut-brain axis in the etiology of autism and propose possible interventions in a preclinical model of autism.
Keywords: BDNF; Bifidobacterium; Clostridium cluster IV; Clostridium perfringens; autism spectrum disorder; fecal transplant; hippocampus; microbiota; propionic acid.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Maenner M.J., Shaw K.A., Baio J., Washington A., Patrick M., DiRienzo M., Christensen D.L., Wiggins L.D., Pettygrove S., Andrews J.G., et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020;69:1–12. doi: 10.15585/mmwr.ss6904a1. - DOI - PMC - PubMed
-
- Hallmayer J., Cleveland S., Torres A., Phillips J., Cohen B., Torigoe T., Miller J., Fedele A., Collins J., Smith K., et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. - DOI - PMC - PubMed
-
- De Angelis M., Piccolo M., Vannini L., Siragusa S., De Giacomo A., Serrazzanetti D.I., Cristofori F., Guerzoni M.E., Gobbetti M., Francavilla R. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS ONE. 2013;8:e76993. doi: 10.1371/journal.pone.0076993. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

