A Modified Meiotic Recombination in Brassica napus Largely Improves Its Breeding Efficiency
- PMID: 34440003
- PMCID: PMC8389541
- DOI: 10.3390/biology10080771
A Modified Meiotic Recombination in Brassica napus Largely Improves Its Breeding Efficiency
Abstract
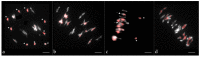
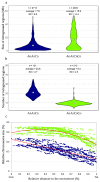
Meiotic recombination is the main tool used by breeders to generate biodiversity, allowing genetic reshuffling at each generation. It enables the accumulation of favorable alleles while purging deleterious mutations. However, this mechanism is highly regulated with the formation of one to rarely more than three crossovers, which are not randomly distributed. In this study, we showed that it is possible to modify these controls in oilseed rape (Brassica napus, AACC, 2n = 4x = 38) and that it is linked to AAC allotriploidy and not to polyploidy per se. To that purpose, we compared the frequency and the distribution of crossovers along A chromosomes from hybrids carrying exactly the same A nucleotide sequence, but presenting three different ploidy levels: AA, AAC and AACC. Genetic maps established with 202 SNPs anchored on reference genomes revealed that the crossover rate is 3.6-fold higher in the AAC allotriploid hybrids compared to AA and AACC hybrids. Using a higher SNP density, we demonstrated that smaller and numerous introgressions of B. rapa were present in AAC hybrids compared to AACC allotetraploid hybrids, with 7.6 Mb vs. 16.9 Mb on average and 21 B. rapa regions per plant vs. nine regions, respectively. Therefore, this boost of recombination is highly efficient to reduce the size of QTL carried in cold regions of the oilseed rape genome, as exemplified here for a QTL conferring blackleg resistance.
Keywords: Brassica napus; allotriploidy; genetic mapping; plant breeding; polyploidy; recombination rate and distribution.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

