Noble Metals for Modern Implant Materials: MOCVD of Film Structures and Cytotoxical, Antibacterial, and Histological Studies
- PMID: 34440054
- PMCID: PMC8389635
- DOI: 10.3390/biomedicines9080851
Noble Metals for Modern Implant Materials: MOCVD of Film Structures and Cytotoxical, Antibacterial, and Histological Studies
Abstract
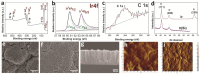
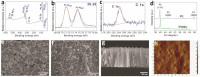
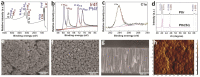
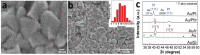
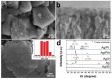
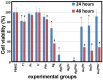
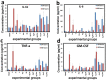
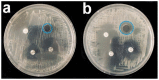
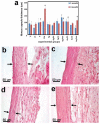
This work is aimed at developing the modification of the surface of medical implants with film materials based on noble metals in order to improve their biological characteristics. Gas-phase transportation methods were proposed to obtain such materials. To determine the effect of the material of the bottom layer of heterometallic structures, Ir, Pt, and PtIr coatings with a thickness of 1.4-1.5 μm were deposited by metal-organic chemical vapor deposition (MOCVD) on Ti6Al4V alloy discs. Two types of antibacterial components, namely, gold nanoparticles (AuNPs) and discontinuous Ag coatings, were deposited on the surface of these coatings. AuNPs (11-14 nm) were deposited by a pulsed MOCVD method, while Ag films (35-40 nm in thickness) were obtained by physical vapor deposition (PVD). The cytotoxic (24 h and 48 h, toward peripheral blood mononuclear cells (PBMCs)) and antibacterial (24 h) properties of monophase (Ag, Ir, Pt, and PtIr) and heterophase (Ag/Pt, Ag/Ir, Ag/PtIr, Au/Pt, Au/Ir, and Au/PtIr) film materials deposited on Ti-alloy samples were studied in vitro and compared with those of uncoated Ti-alloy samples. Studies of the cytokine production by PBMCs in response to incubation of the samples for 24 and 48 h and histological studies at 1 and 3 months after subcutaneous implantation in rats were also performed. Despite the comparable thickness of the fibrous capsule after 3 months, a faster completion of the active phase of encapsulation was observed for the coated implants compared to the Ti alloy analogs. For the Ag-containing samples, growth inhibition of S. epidermidis, S. aureus, Str. pyogenes, P. aeruginosa, and Ent. faecium was observed.
Keywords: biochemical and cytokine blood composition; chemical vapor deposition; cytological; gold; histological study; iridium; platinum; silver; thin films and nanoparticles; titanium-alloy implants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Basova T.V., Vikulova E.S., Dorovskikh S.I., Hassan A., Morozova N.B. The use of noble metal coatings and nanoparticles for the modification of medical implant materials. Mater. Des. 2021;204:109672. doi: 10.1016/j.matdes.2021.109672. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

