Local Delivery of Pirfenidone by PLA Implants Modifies Foreign Body Reaction and Prevents Fibrosis
- PMID: 34440057
- PMCID: PMC8389617
- DOI: 10.3390/biomedicines9080853
Local Delivery of Pirfenidone by PLA Implants Modifies Foreign Body Reaction and Prevents Fibrosis
Abstract
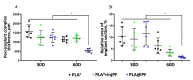
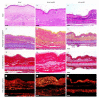
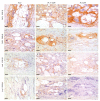
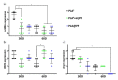
Peri-implant fibrosis (PIF) increases the postsurgical risks after implantation and limits the efficacy of the implantable drug delivery systems (IDDS). Pirfenidone (PF) is an oral anti-fibrotic drug with a short (<3 h) circulation half-life and strong adverse side effects. In the current study, disk-shaped IDDS prototype combining polylactic acid (PLA) and PF, PLA@PF, with prolonged (~3 days) PF release (in vitro) was prepared. The effects of the PLA@PF implants on PIF were examined in the rabbit ear skin pocket model on postoperative days (POD) 30 and 60. Matching blank PLA implants (PLA0) and PLA0 with an equivalent single-dose PF injection performed on POD0 (PLA0+injPF) served as control. On POD30, the intergroup differences were observed in α-SMA, iNOS and arginase-1 expressions in PLA@PF and PLA0+injPF groups vs. PLA0. On POD60, PIF was significantly reduced in PLA@PF group. The peri-implant tissue thickness decreased (532 ± 98 μm vs. >1100 μm in control groups) approaching the intact derma thickness value (302 ± 15 μm). In PLA@PF group, the implant biodegradation developed faster, while arginase-1 expression was suppressed in comparison with other groups. This study proves the feasibility of the local control of fibrotic response on implants via modulation of foreign body reaction with slowly biodegradable PF-loaded IDDS.
Keywords: anti-fibrotic therapy; collagen; fibrosis; foreign body reaction; implantable drug delivery systems; peri-implant fibrosis; pirfenidone; polylactic acid; polymer implants; quantitative histopathology.
Conflict of interest statement
The authors declare no conflict of interests.
Figures













References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

