Local Inhibition of Indoleamine 2,3-Dioxygenase Mitigates Renal Fibrosis
- PMID: 34440060
- PMCID: PMC8389588
- DOI: 10.3390/biomedicines9080856
Local Inhibition of Indoleamine 2,3-Dioxygenase Mitigates Renal Fibrosis
Abstract
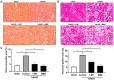
Chronic kidney disease (CKD) is a major global health concern and renal fibrosis is an integral part of the pathophysiological mechanism underlying disease progression. In CKD patients, the majority of metabolic pathways are in disarray and perturbations in enzyme activity most likely contribute to the wide variety of comorbidities observed in these patients. To illustrate, catabolism of tryptophan by indoleamine 2,3-dioxygenase (IDO) gives rise to numerous biologically active metabolites implicated in CKD progression. Here, we evaluated the effect of antagonizing IDO on renal fibrogenesis. To this end, we antagonized IDO using 1-methyl-D-tryptophan (1-MT) and BMS-98620 in TGF-β-treated murine precision-cut kidney slices (mPCKS) and in mice subjected to unilateral ureteral obstruction (UUO). The fibrotic response was evaluated on both the gene and protein level using qPCR and western blotting. Our results demonstrated that treatment with 1-MT or BMS-985205 markedly reduced TGF-β-mediated fibrosis in mPCKS, as seen by a decreased expression of collagen type 1, fibronectin, and α-smooth muscle actin. Moreover, IDO protein expression clearly increased following UUO, however, treatment of UUO mice with either 1-MT or BMS-986205 did not significantly affect the gene and protein expression of the tested fibrosis markers. However, both inhibitors significantly reduced the renal deposition of collagen in UUO mice as shown by Sirius red and trichrome staining. In conclusion, this study demonstrates that IDO antagonism effectively mitigates fibrogenesis in mPCKS and reduces renal collagen accumulation in UUO mice. These findings warrant further research into the clinical application of IDO inhibitors for the treatment of renal fibrosis.
Keywords: 1-methyl-tryptophan; BMS-98620; indoleamine 2,3-dioxygenase; precision-cut kidney slices; renal fibrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Schefold J.C., Zeden J.-P., Fotopoulou C., Von Haehling S., Pschowski R., Hasper D., Volk H.-D., Schuett C., Reinke P. Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: A possible link between chronic inflammation and uraemic symptoms. Nephrol. Dial. Transplant. 2009;24:1901–1908. doi: 10.1093/ndt/gfn739. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

