Au2phen and Auoxo6, Two Dinuclear Oxo-Bridged Gold(III) Compounds, Induce Apoptotic Signaling in Human Ovarian A2780 Cancer Cells
- PMID: 34440075
- PMCID: PMC8389655
- DOI: 10.3390/biomedicines9080871
Au2phen and Auoxo6, Two Dinuclear Oxo-Bridged Gold(III) Compounds, Induce Apoptotic Signaling in Human Ovarian A2780 Cancer Cells
Abstract
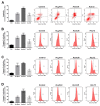
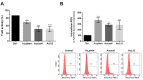
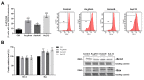
Au2phen ((2,9-dimethyl-1,10-phenanthroline)2Au2(µ-O)2)(PF6)2 and Auoxo6 ((6,6'-dimethyl-2,2'-bipyridine)2Au2(µ-O)2)(PF6)2 are two structurally related gold(III) complexes that were previously reported to display relevant and promising anticancer properties in vitro toward a large number of human cancer cell lines. To expand the knowledge on the molecular mechanisms through which these gold(III) complexes trigger apoptosis in cancer cells, further studies have been performed using A2780 ovarian cancer cells as reference models. For comparative purposes, parallel studies were carried out on the gold(III) complex AuL12 (dibromo(ethylsarcosinedithiocarbamate)gold(III)), whose proapoptotic profile had been earlier characterized in several cancer cell lines. Our results pointed out that all these gold(III) compounds manifest a significant degree of similarity in their cellular and proapoptotic effects; the main observed perturbations consist of potent thioredoxin reductase inhibition, disruption of the cell redox balance, impairment of the mitochondrial membrane potential, and induction of associated metabolic changes. In addition, evidence was gained of the remarkable contribution of ASK1 (apoptosis-signal-regulating kinase-1) and AKT pathways to gold(III)-induced apoptotic signaling. Overall, the observed effects may be traced back to gold(III) reduction and subsequent formation and release of gold(I) species that are able to bind and inhibit several enzymes responsible for the intracellular redox homeostasis, in particular the selenoenzyme thioredoxin reductase.
Keywords: A2780 ovarian cancer cells; apoptosis signal pathway; gold(III)-based compounds; mitochondria; thioredoxin reductase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Casini A., Kelter G., Gabbiani C., Cinellu M.A., Minghetti G., Fregona D., Fiebig H.-H., Messori L. Chemistry, Antiproliferative Properties, Tumor Selectivity, and Molecular Mechanisms of Novel Gold(III) Compounds for Cancer Treatment: A Systematic Study. JBIC J. Biol. Inorg. Chem. 2009;14:1139–1149. doi: 10.1007/s00775-009-0558-9. - DOI - PubMed
-
- Aldinucci D., Ronconi L., Fregona D. Groundbreaking Gold(III) Anticancer Agents. Drug Discov. Today. 2009;14:1075–1076. doi: 10.1016/j.drudis.2009.07.011. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

