The Roles of Post-Translational Modifications in STAT3 Biological Activities and Functions
- PMID: 34440160
- PMCID: PMC8393524
- DOI: 10.3390/biomedicines9080956
The Roles of Post-Translational Modifications in STAT3 Biological Activities and Functions
Abstract
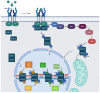
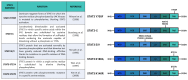
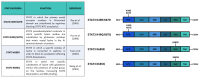
STAT3 is an important transcription factor that regulates cell growth and proliferation by regulating gene transcription of a plethora of genes. This protein also has many roles in cancer progression and several tumors such as prostate, lung, breast, and intestine cancers that are characterized by strong STAT3-dependent transcriptional activity. This protein is post-translationally modified in different ways according to cellular context and stimulus, and the same post-translational modification can have opposite effects in different cellular models. In this review, we describe the studies performed on the main modifications affecting the activity of STAT3: phosphorylation of tyrosine 705 and serine 727; acetylation of lysine 49, 87, 601, 615, 631, 685, 707, and 709; and methylation of lysine 49, 140, and 180. The extensive results obtained by different studies demonstrate that post-translational modifications drastically change STAT3 activities and that we need further analysis to properly elucidate all the functions of this multifaceted transcription factor.
Keywords: STAT3; acetylation; methylation; phosphorylation; post-translational modifications.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

