Non-Coding RNA-Based Biosensors for Early Detection of Liver Cancer
- PMID: 34440168
- PMCID: PMC8391662
- DOI: 10.3390/biomedicines9080964
Non-Coding RNA-Based Biosensors for Early Detection of Liver Cancer
Abstract
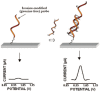
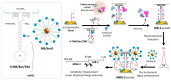
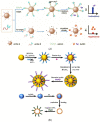
Primary liver cancer is an aggressive, lethal malignancy that ranks as the fourth leading cause of cancer-related death worldwide. Its 5-year mortality rate is estimated to be more than 95%. This significant low survival rate is due to poor diagnosis, which can be referred to as the lack of sufficient and early-stage detection methods. Many liver cancer-associated non-coding RNAs (ncRNAs) have been extensively examined to serve as promising biomarkers for precise diagnostics, prognostics, and the evaluation of the therapeutic progress. For the simple, rapid, and selective ncRNA detection, various nanomaterial-enhanced biosensors have been developed based on electrochemical, optical, and electromechanical detection methods. This review presents ncRNAs as the potential biomarkers for the early-stage diagnosis of liver cancer. Moreover, a comprehensive overview of recent developments in nanobiosensors for liver cancer-related ncRNA detection is provided.
Keywords: biosensors; electrochemical; electromechanical; liver cancer; nanomaterials; non-coding RNAs (ncRNAs); optical.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

