HMP-S7 Is a Novel Anti-Leukemic Peptide Discovered from Human Milk
- PMID: 34440185
- PMCID: PMC8394283
- DOI: 10.3390/biomedicines9080981
HMP-S7 Is a Novel Anti-Leukemic Peptide Discovered from Human Milk
Abstract
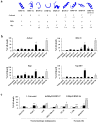
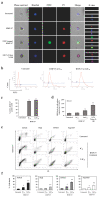
Chemotherapy in childhood leukemia is associated with late morbidity in leukemic survivors, while certain patient subsets are relatively resistant to standard chemotherapy. It is therefore important to identify new agents with sensitivity and selectivity towards leukemic cells, while having less systemic toxicity. Peptide-based therapeutics has gained a great deal of attention during the last few years. Here, we used an integrative workflow combining mass spectrometric peptide library construction, in silico anticancer peptide screening, and in vitro leukemic cell studies to discover a novel anti-leukemic peptide having 3+ charges and an alpha helical structure, namely HMP-S7, from human breast milk. HMP-S7 showed cytotoxic activity against four distinct leukemic cell lines in a dose-dependent manner but had no effect on solid malignancies or representative normal cells. HMP-S7 induced leukemic cell death by penetrating the plasma membrane to enter the cytoplasm and cause the leakage of lactate dehydrogenase, thus acting in a membranolytic manner. Importantly, HMP-S7 exhibited anti-leukemic effects against patient-derived leukemic cells ex vivo. In conclusion, HMP-S7 is a selective anti-leukemic peptide with promise, which requires further validation in preclinical and clinical studies.
Keywords: anticancer peptide; drug discovery; human milk; leukemia; machine learning; mass spectrometry; peptidomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Mulrooney D.A., Hyun G., Ness K.K., Bhakta N., Pui C.H., Ehrhardt M.J., Krull K.R., Crom D.B., Chemaitilly W., Srivastava D.K., et al. The changing burden of long-term health outcomes in survivors of childhood acute lymphoblastic leukaemia: A retrospective analysis of the St Jude Lifetime Cohort Study. Lancet Haematol. 2019;6:e306–e316. doi: 10.1016/S2352-3026(19)30050-X. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

