Differentiation Behaviour of Adipose-Derived Stromal Cells (ASCs) Seeded on Polyurethane-Fibrin Scaffolds In Vitro and In Vivo
- PMID: 34440186
- PMCID: PMC8391877
- DOI: 10.3390/biomedicines9080982
Differentiation Behaviour of Adipose-Derived Stromal Cells (ASCs) Seeded on Polyurethane-Fibrin Scaffolds In Vitro and In Vivo
Abstract
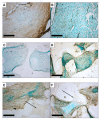
Adipose-derived stromal cells (ASCs) are a promising cell source for tissue engineering and regenerative medicine approaches for cartilage replacement. For chondrogenic differentiation, human (h)ASCs were seeded on three-dimensional polyurethane (PU) fibrin composites and induced with a chondrogenic differentiation medium containing TGF-ß3, BMP-6, and IGF-1 in various combinations. In addition, in vitro predifferentiated cell-seeded constructs were implanted into auricular cartilage defects of New Zealand White Rabbits for 4 and 12 weeks. Histological, immunohistochemical, and RT-PCR analyses were performed on the constructs maintained in vitro to determine extracellular matrix (ECM) deposition and expression of specific cartilage markers. Chondrogenic differentiated constructs showed a uniform distribution of cells and ECM proteins. RT-PCR showed increased gene expression of collagen II, collagen X, and aggrecan and nearly stable expression of SOX-9 and collagen I. Rabbit (r)ASC-seeded PU-fibrin composites implanted in ear cartilage defects of New Zealand White Rabbits showed deposition of ECM with structures resembling cartilage lacunae by Alcian blue staining. However, extracellular calcium deposition became detectable over the course of 12 weeks. RT-PCR showed evidence of endochondral ossification during the time course with the expression of specific marker genes (collagen X and RUNX-2). In conclusion, hASCs show chondrogenic differentiation capacity in vitro with the expression of specific marker genes and deposition of cartilage-specific ECM proteins. After implantation of predifferentiated rASC-seeded PU-fibrin scaffolds into a cartilage defect, the constructs undergo the route of endochondral ossification.
Keywords: ASC; BMP-6; TGF-ß3; adipose-derived stromal cells; chondrogenic differentiation; endochondral ossification; fibrin; polyurethane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

