Multiple-Organ Complement Deposition on Vascular Endothelium in COVID-19 Patients
- PMID: 34440207
- PMCID: PMC8394811
- DOI: 10.3390/biomedicines9081003
Multiple-Organ Complement Deposition on Vascular Endothelium in COVID-19 Patients
Abstract
Increased levels of circulating complement activation products have been reported in COVID-19 patients, but only limited information is available on complement involvement at the tissue level. The mechanisms and pathways of local complement activation remain unclear. The aim of this study was to investigate the deposition of complement components in the lungs, kidneys, and liver in patients with COVID-19 patients and to determine the pathway/s of complement activation. We performed immunofluorescence analyses of autopsy specimens of lungs, kidney, and liver from 12 COVID-19 patients who died of acute respiratory failure. Snap-frozen samples embedded in OCT were stained with antibodies against complement components and activation products, IgG, and spike protein of SARS-CoV-2. Lung deposits of C1q, C4, C3, and C5b-9 were localized in the capillaries of the interalveolar septa and on alveolar cells. IgG displayed a similar even distribution, suggesting classical pathway activation. The spike protein is a potential target of IgG, but its uneven distribution suggests that other viral and tissue molecules may be targeted by IgG. FB deposits were also seen in COVID-19 lungs and are consistent with activation of the alternative pathway, whereas MBL and MASP-2 were hardly detectable. Analysis of kidney and liver specimens mirrored findings observed in the lung. Complement deposits were seen on tubules and vessels of the kidney with only mild C5b-9 staining in glomeruli, and on the hepatic artery and portal vein of the liver. Complement deposits in different organs of deceased COVID-19 patients caused by activation of the classical and alternative pathways support the multi-organ nature of the disease and the contribution of the complement system to inflammation and tissue damage.
Keywords: COVID-19; classical pathway; complement activation; multi-organ deposition; spike protein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
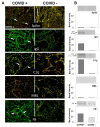

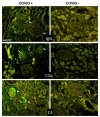

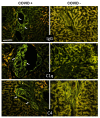
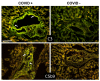
Update of
-
Multi-organ complement deposition in COVID-19 patients.medRxiv [Preprint]. 2021 Jan 8:2021.01.07.21249116. doi: 10.1101/2021.01.07.21249116. medRxiv. 2021. Update in: Biomedicines. 2021 Aug 12;9(8):1003. doi: 10.3390/biomedicines9081003. PMID: 33442701 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

