The Combined Effect of Branching and Elongation on the Bioactivity Profile of Phytocannabinoids. Part I: Thermo-TRPs
- PMID: 34440274
- PMCID: PMC8391922
- DOI: 10.3390/biomedicines9081070
The Combined Effect of Branching and Elongation on the Bioactivity Profile of Phytocannabinoids. Part I: Thermo-TRPs
Abstract
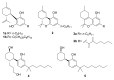
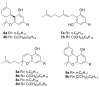
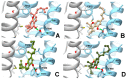
The affinity of cannabinoids for their CB1 and CB2 metabotropic receptors is dramatically affected by a combination of α-branching and elongation of their alkyl substituent, a maneuver exemplified by the n-pentyl -> α,α-dimethylheptyl (DMH) swap. The effect of this change on other cannabinoid end-points is still unknown, an observation surprising since thermo-TRPs are targeted by phytocannabinoids with often sub-micromolar affinity. To fill this gap, the α,α-dimethylheptyl analogues of the five major phytocannabinoids [CBD (1a), Δ8-THC (6a), CBG (7a), CBC (8a) and CBN (9a)] were prepared by total synthesis, and their activity on thermo-TRPs (TRPV1-4, TRPM8, and TRPA1) was compared with that of one of their natural analogues. Surprisingly, the DMH chain promoted a shift in the selectivity toward TRPA1, a target involved in pain and inflammatory diseases, in all investigated compounds. A comparative study of the putative binding modes at TRPA1 between DMH-CBC (8b), the most active compound within the series, and CBC (8a) was carried out by molecular docking, allowing the rationalization of their activity in terms of structure-activity relationships. Taken together, these observations qualify DMH-CBC (8b) as a non-covalent TRPA1-selective cannabinoid lead that is worthy of additional investigation as an analgesic and anti-inflammatory agent.
Keywords: TRPA1; cannabichromene; phytocannabinoids; thermos-TRPs; α,α-dimethylheptyl effect.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Appendino G. The early history of cannabinoid research. Rend. Fis. Acc. Lincei. 2020;31:919–929. doi: 10.1007/s12210-020-00956-0. - DOI
-
- Gaoni Y., Mechoulam R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964;86:1646–1647. doi: 10.1021/ja01062a046. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

