Intracellular Development of Resident Cardiac Stem Cells: An Overlooked Phenomenon in Myocardial Self-Renewal and Regeneration
- PMID: 34440467
- PMCID: PMC8399953
- DOI: 10.3390/life11080723
Intracellular Development of Resident Cardiac Stem Cells: An Overlooked Phenomenon in Myocardial Self-Renewal and Regeneration
Abstract
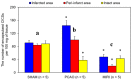
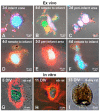
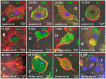
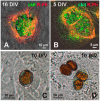
At present, the approaches aimed at increasing myocardial regeneration after infarction are not available. The key question is the identity of cells capable of producing functional cardiac myocytes (CMs), replenishing those lost during ischemia. With identification of resident cardiac stem cells (CSCs), it has been supposed that this cell population may be crucial for myocardial self-renewal and regeneration. In the last few years, the focus has been shifted towards another concept, implying that new CMs are produced by dedifferentiation and proliferation of mature CMs. The observation that CSCs can undergo development inside immature cardiac cells by formation of "cell-in-cell structures" (CICSs) has enabled us to conclude that encapsulated CICSs are implicated in mammalian cardiomyogenesis over the entire lifespan. Earlier we demonstrated that new CMs are produced through formation of CSC-derived transitory amplifying cells (TACs) either in the CM colonies or inside encapsulated CICSs. In this study, we described the phenomenon of CSC penetration into mature CMs, resulting in the formation of vacuole-like CICSs (or non-encapsulated CICSs) containing proliferating CSCs with subsequent differentiation of CSC progeny into TACs and their release. In addition, we compared the phenotypes of TACs derived from encapsulated and non-encapsulated CICSs developing in immature and mature CMs, respectively.
Keywords: cardiac stem cells (CSCs); cardiomyocytes (CMs); cardiomyogenesis; myocardial ischemia; regeneration; transitory amplifying cells (TACs); “cell-in-cell structures” (CICSs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
- No 12-04-00941 and 16-04-01424/the Russian Foundation for Basic Research
- 2012-2014/"Fundamental Sciences for Medicine" (2012-2014)
- 2014-2018/FASO of Russia ("The mechanisms of development of neuropsychic, metabolic, and hormonal dysfunctions in the nervous and endocrine diseases and the approaches to their correction")
LinkOut - more resources
Full Text Sources

