Protective Effects of Probiotics on Cognitive and Motor Functions, Anxiety Level, Visceral Sensitivity, Oxidative Stress and Microbiota in Mice with Antibiotic-Induced Dysbiosis
- PMID: 34440509
- PMCID: PMC8398215
- DOI: 10.3390/life11080764
Protective Effects of Probiotics on Cognitive and Motor Functions, Anxiety Level, Visceral Sensitivity, Oxidative Stress and Microbiota in Mice with Antibiotic-Induced Dysbiosis
Abstract
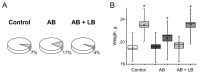
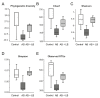
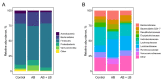
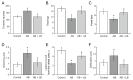
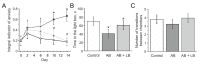
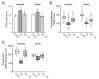
Accumulating clinical and preclinical data indicate a prominent role of gut microbiota in regulation of physiological functions. The gut-brain axis imbalance due to gut dysbiosis is associated with a range of neurodegenerative diseases. Probiotics were suggested not only to restore intestinal dysbiosis but also modulate stress response and improve mood and anxiety symptoms. In this study, we assessed the effects of probiotic lactobacilli on behavioral reactions, the level of oxidative stress and microbiota content in mice administered to broad-spectrum antibiotics. Our study demonstrates that antibiotic treatment of adolescent mice for two weeks resulted in higher mortality and lower weight gain and induced significant changes in behavior including lower locomotor and exploratory activity, reduced muscle strength, visceral hypersensitivity, higher level of anxiety and impaired cognitive functions compared to the control group. These changes were accompanied by decreased diversity and total amount of bacteria, abundance of Proteobacteria and Verrucomicrobia phyla, and reduced Firmicutes/Bacteroides ratio in the gut microbiota. Moreover, a higher level of oxidative stress was found in brain and skeletal muscle tissues of mice treated with antibiotics. Oral administration of two Lactobacillus strains prevented the observed changes and improved not only microbiota content but also the behavioral alterations, suggesting a neuroprotective and antioxidant role of probiotics.
Keywords: antibiotic-induced dysbiosis; anxiety level; cognitive functions; locomotor and exploratory activity; microbiota; motor coordination; muscle strength; oxidative stress; probiotic lactobacilli; visceral and mechanical sensitivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Pokrovskaya E.V., Shamkhalova M.S., Shestakova M.V. The new views on the state of the gut microbiota in obesity and diabetes mellitus type 2. Review. Diabetes Mellit. 2019;22:253–262. doi: 10.14341/DM10194. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

