Translating Treg Therapy for Inflammatory Bowel Disease in Humanized Mice
- PMID: 34440615
- PMCID: PMC8393385
- DOI: 10.3390/cells10081847
Translating Treg Therapy for Inflammatory Bowel Disease in Humanized Mice
Abstract
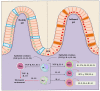
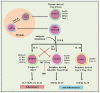
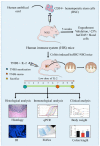
Crohn's disease and ulcerative colitis, two major forms of inflammatory bowel disease (IBD) in humans, afflicted in genetically predisposed individuals due to dysregulated immune response directed against constituents of gut flora. The defective immune responses mounted against the regulatory mechanisms amplify and maintain the IBD-induced mucosal inflammation. Therefore, restoring the balance between inflammatory and anti-inflammatory immunepathways in the gut may contribute to halting the IBD-associated tissue-damaging immune response. Phenotypic and functional characterization of various immune-suppressive T cells (regulatory T cells; Tregs) over the last decade has been used to optimize the procedures for in vitro expansion of these cells for developing therapeutic interventional strategies. In this paper, we review the mechanisms of action and functional importance of Tregs during the pathogenesis of IBD and modulating the disease induced inflammation as well as role of mouse models including humanized mice repopulated with the human immune system (HIS) to study the IBD. "Humanized" mouse models provide new tools to analyze human Treg ontogeny, immunobiology, and therapy and the role of Tregs in developing interventional strategies against IBD. Overall, humanized mouse models replicate the human conditions and prove a viable tool to study molecular functions of human Tregs to harness their therapeutic potential.
Keywords: Crohn’s disease; human immune system; humanized mice; inflammatory bowels disease; regulatory T cells; ulcerative colitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

