Inflammation and Aging of Hematopoietic Stem Cells in Their Niche
- PMID: 34440618
- PMCID: PMC8391820
- DOI: 10.3390/cells10081849
Inflammation and Aging of Hematopoietic Stem Cells in Their Niche
Abstract
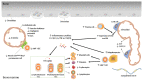
Hematopoietic stem cells (HSCs) sustain the lifelong production of all blood cell lineages. The functioning of aged HSCs is impaired, including a declined repopulation capacity and myeloid and platelet-restricted differentiation. Both cell-intrinsic and microenvironmental extrinsic factors contribute to HSC aging. Recent studies highlight the emerging role of inflammation in contributing to HSC aging. In this review, we summarize the recent finding of age-associated changes of HSCs and the bone marrow niche in which they lodge, and discuss how inflammation may drive HSC aging.
Keywords: aging; hematopoietic stem cells; heterogeneity; inflammation; niche.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Frisch B.J., Hoffman C.M., Latchney S.E., LaMere M.W., Myers J., Ashton J., Li A.J., Saunders J., Palis J., Perkins A.S., et al. Aged marrow macrophages expand platelet-biased hematopoietic stem cells via interleukin-1B. JCI Insight. 2019;4:e124213. doi: 10.1172/jci.insight.124213. - DOI - PMC - PubMed
-
- Ho Y.H., del Toro R., Rivera-Torres J., Rak J., Korn C., García-García A., Macías D., González-Gómez C., del Monte A., Wittner M., et al. Remodeling of Bone Marrow Hematopoietic Stem Cell Niches Promotes Myeloid Cell Expansion during Premature or Physiological Aging. Cell Stem Cell. 2019;25:407–418.e6. doi: 10.1016/j.stem.2019.06.007. - DOI - PMC - PubMed
-
- Grover A., Sanjuan-Pla A., Thongjuea S., Carrelha J., Giustacchini A., Gambardella A., Macaulay I., Mancini E., Luis T.C., Mead A., et al. Single-cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nat. Commun. 2016;7:1–12. doi: 10.1038/ncomms11075. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

