Multisystemic Cellular Tropism of SARS-CoV-2 in Autopsies of COVID-19 Patients
- PMID: 34440669
- PMCID: PMC8394956
- DOI: 10.3390/cells10081900
Multisystemic Cellular Tropism of SARS-CoV-2 in Autopsies of COVID-19 Patients
Abstract
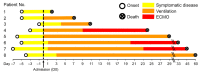
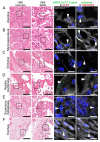
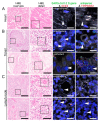
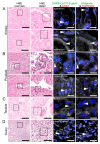
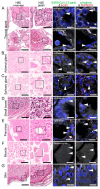
Multiorgan tropism of SARS-CoV-2 has previously been shown for several major organs. We have comprehensively analyzed 25 different formalin-fixed paraffin-embedded (FFPE) tissues/organs from autopsies of fatal COVID-19 cases (n = 8), using histopathological assessment, detection of SARS-CoV-2 RNA using polymerase chain reaction and RNA in situ hybridization, viral protein using immunohistochemistry, and virus particles using transmission electron microscopy. SARS-CoV-2 RNA was mainly localized in epithelial cells across all organs. Next to lung, trachea, kidney, heart, or liver, viral RNA was also found in tonsils, salivary glands, oropharynx, thyroid, adrenal gland, testicles, prostate, ovaries, small bowel, lymph nodes, skin and skeletal muscle. Viral RNA was predominantly found in cells expressing ACE2, TMPRSS2, or both. The SARS-CoV-2 replicating RNA was also detected in these organs. Immunohistochemistry and electron microscopy were not suitable for reliable and specific SARS-CoV-2 detection in autopsies. These findings were validated using in situ hybridization on external COVID-19 autopsy samples (n = 9). Apart from the lung, correlation of viral detection and histopathological assessment did not reveal any specific alterations that could be attributed to SARS-CoV-2. In summary, SARS-CoV-2 and its replication could be observed across all organ systems, which co-localizes with ACE2 and TMPRSS2 mainly in epithelial but also in mesenchymal and endothelial cells. Apart from the respiratory tract, no specific (histo-)morphologic alterations could be assigned to the SARS-CoV-2 infection.
Keywords: ACE2; SARS-CoV-2; TMPRSS2; formalin-fixed paraffin-embedded (FFPE) tissue; histology; post-mortem.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

