Processing and Ex Vivo Expansion of Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells for the Development of an Advanced Therapy Medicinal Product for use in Humans
- PMID: 34440677
- PMCID: PMC8392403
- DOI: 10.3390/cells10081908
Processing and Ex Vivo Expansion of Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells for the Development of an Advanced Therapy Medicinal Product for use in Humans
Abstract
Adipose tissue (AT) represents a commonly used source of mesenchymal stem/stromal cells (MSCs) whose proregenerative potential has been widely investigated in multiple clinical trials worldwide. However, the standardization of the manufacturing process of MSC-based cell therapy medicinal products in compliance with the requirements of the local authorities is obligatory and will allow us to obtain the necessary permits for product administration according to its intended use. Within the research phase (RD), we optimized the protocols used for the processing and ex vivo expansion of AT-derived MSCs (AT-MSCs) for the development of an Advanced Therapy Medicinal Product (ATMP) for use in humans. Critical process parameters (including, e.g., the concentration of enzyme used for AT digestion, cell culture conditions) were identified and examined to ensure the high quality of the final product containing AT-MSCs. We confirmed the identity of isolated AT-MSCs as MSCs and their trilineage differentiation potential according to the International Society for Cellular Therapy (ISCT) recommendations. Based on the conducted experiments, in-process quality control (QC) parameters and acceptance criteria were defined for the manufacturing of hospital exemption ATMP (HE-ATMP). Finally, we conducted a validation of the manufacturing process in a GMP facility. In the current study, we presented a process approach leading to the optimization of processing and the ex vivo expansion of AT-MSCs for the development of ATMP for use in humans.
Keywords: adipose tissue-derived mesenchymal stem/stromal cells; advanced therapy medicinal product; cell-based therapy; good manufacturing practices; regenerative medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

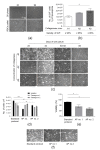
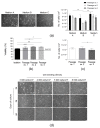

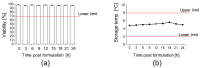
References
-
- Labedz-Maslowska A., Bryniarska N., Kubiak A., Kaczmarzyk T., Sekula-Stryjewska M., Noga S., Boruczkowski D., Madeja Z., Zuba-Surma E. Multilineage differentiation potential of human dental pulp stem cells—Impact of 3d and hypoxic environment on osteogenesis in vitro. Int. J. Mol. Sci. 2020;21:6172. doi: 10.3390/ijms21176172. - DOI - PMC - PubMed
-
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S., Deans R.J., Keating A., Prockop D.J., Horwitz E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

