Identification of Novel Cathepsin B Inhibitors with Implications in Alzheimer's Disease: Computational Refining and Biochemical Evaluation
- PMID: 34440715
- PMCID: PMC8391575
- DOI: 10.3390/cells10081946
Identification of Novel Cathepsin B Inhibitors with Implications in Alzheimer's Disease: Computational Refining and Biochemical Evaluation
Abstract
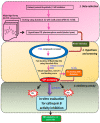
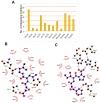
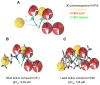
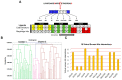
Amyloid precursor protein (APP), upon proteolytic degradation, forms aggregates of amyloid β (Aβ) and plaques in the brain, which are pathological hallmarks of Alzheimer's disease (AD). Cathepsin B is a cysteine protease enzyme that catalyzes the proteolytic degradation of APP in the brain. Thus, cathepsin B inhibition is a crucial therapeutic aspect for the discovery of new anti-Alzheimer's drugs. In this study, we have employed mixed-feature ligand-based virtual screening (LBVS) by integrating pharmacophore mapping, docking, and molecular dynamics to detect small, potent molecules that act as cathepsin B inhibitors. The LBVS model was generated by using hydrophobic (HY), hydrogen bond acceptor (HBA), and hydrogen bond donor (HBD) features, using a dataset of 24 known cathepsin B inhibitors of both natural and synthetic origins. A validated eight-feature pharmacophore hypothesis (Hypo III) was utilized to screen the Maybridge chemical database. The docking score, MM-PBSA, and MM-GBSA methodology was applied to prioritize the lead compounds as virtual screening hits. These compounds share a common amide scaffold, and showed important interactions with Gln23, Cys29, His110, His111, Glu122, His199, and Trp221. The identified inhibitors were further evaluated for cathepsin-B-inhibitory activity. Our study suggests that pyridine, acetamide, and benzohydrazide compounds could be used as a starting point for the development of novel therapeutics.
Keywords: 3D pharmacophore; Alzheimer’s disease; cathepsin B; docking; molecular dynamics; virtual screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bogdanovic N., Hansson O., Zetterberg H., Basun H., Ingelsson M., Lannfelt L., Blennow K. Alzheimer’s disease—The most common cause of dementia. Lakartidningen. 2020;117 - PubMed
-
- Marelli C., Hourregue C., Gutierrez L.A., Paquet C., de Menjot Champfleur N., De Verbizier D., Jacob M., Dubois J., Maleska A.M., Hirtz C., et al. Cerebrospinal Fluid and Plasma Biomarkers do not Differ in the Presenile and Late-Onset Behavioral Variants of Frontotemporal Dementia. J. Alzheimers Dis. 2020;74:903–911. doi: 10.3233/JAD-190378. - DOI - PubMed
-
- Gaugler J., James B., Johnson T., Marin A., Weuve J., Assoc A.S. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15:321–387. doi: 10.1016/j.jalz.2019.01.010. - DOI
-
- Gupta V., Gupta V.B., Chitranshi N., Gangoda S., Vander Wall R., Abbasi M., Golzan M., Dheer Y., Shah T., Avolio A., et al. One protein, multiple pathologies: Multifaceted involvement of amyloid beta in neurodegenerative disorders of the brain and retina. Cell Mol. Life Sci. 2016;73:4279–4297. doi: 10.1007/s00018-016-2295-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

