Immunomodulation and Biomaterials: Key Players to Repair Volumetric Muscle Loss
- PMID: 34440785
- PMCID: PMC8394423
- DOI: 10.3390/cells10082016
Immunomodulation and Biomaterials: Key Players to Repair Volumetric Muscle Loss
Abstract
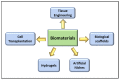
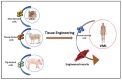
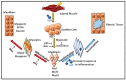
Volumetric muscle loss (VML) is defined as a condition in which a large volume of skeletal muscle is lost due to physical insult. VML often results in a heightened immune response, resulting in significant long-term functional impairment. Estimates indicate that ~250,000 fractures occur in the US alone that involve VML. Currently, there is no active treatment to fully recover or repair muscle loss in VML patients. The health economics burden due to VML is rapidly increasing around the world. Immunologists, developmental biologists, and muscle pathophysiologists are exploring both immune responses and biomaterials to meet this challenging situation. The inflammatory response in muscle injury involves a non-specific inflammatory response at the injured site that is coordination between the immune system, especially macrophages and muscle. The potential role of biomaterials in the regenerative process of skeletal muscle injury is currently an important topic. To this end, cell therapy holds great promise for the regeneration of damaged muscle following VML. However, the delivery of cells into the injured muscle site poses a major challenge as it might cause an adverse immune response or inflammation. To overcome this obstacle, in recent years various biomaterials with diverse physical and chemical nature have been developed and verified for the treatment of various muscle injuries. These biomaterials, with desired tunable physicochemical properties, can be used in combination with stem cells and growth factors to repair VML. In the current review, we focus on how various immune cells, in conjunction with biomaterials, can be used to promote muscle regeneration and, most importantly, suppress VML pathology.
Keywords: VML; biomaterials; immune response; reconstructive therapies.
Conflict of interest statement
The authors declare not having any competing interest that might influence this study or no involvement of study sponsors.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

