Physcion Induces Potential Anticancer Effects in Cervical Cancer Cells
- PMID: 34440797
- PMCID: PMC8392222
- DOI: 10.3390/cells10082029
Physcion Induces Potential Anticancer Effects in Cervical Cancer Cells
Abstract
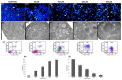
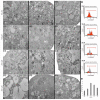
Background: The extent of morphological and ultrastructural changes in HeLa cells was assessed by optical, fluorescence and electron microscopy after exposure to various concentrations of physcion, taking into account the biological properties of the test compound.
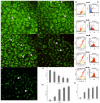
Methods: Cell viability was assessed by MTT assay, while the cell cycle, LC3 expression, apoptosis, change of mitochondrial potential, Bcl-2 protein expression level and the level of reactive oxygen species were analyzed by flow cytometry.
Results: As a result of physcion encumbrance, concentration-dependent inhibition of HeLa cell viability and the G0/G1 phase of the cell cycle was observed. Activation of the lysosomal system was also revealed, which was expressed by an increased number of lysosomes, autophage vacuoles and increased expression of the LC3 protein, a marker of the autophagy process. Transmission electron microscopy and fluorescence microscopy showed that physcion induced clear changes in cervical cancer cells, especially in the structure of the nucleus and mitochondria, which correlated with the production of reactive oxygen species by the test compound and indicated the induction of the oxidative process. At the same time, the pro-apoptotic effect of physcion was demonstrated, and this mechanism was dependent on the activation of caspases 3/7 and the reduction in Bcl-2 protein expression.
Conclusion: The obtained results indicate an antitumor mechanism of action of physcion, based on the induction of oxidative stress, autophagy and apoptosis.
Keywords: apoptosis; autophagy; mitochondria; oxidative stress; physcion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Król T., Schmidt M., Kołątaj A., Witek B. Vinblastine-induced autophagy in mouse liver. Comp. Biochem. Physiol. C Toxicol. Pharm. 1994;107:165–169. doi: 10.1016/1367-8280(94)90023-X. - DOI
-
- Król T. Vincristine-induced autophagy in mouse liver. Acta Biol. Cracoviensia. Ser. Zool. 1996;38:5–12.

