NAFLD-Related Hepatocarcinoma: The Malignant Side of Metabolic Syndrome
- PMID: 34440803
- PMCID: PMC8391372
- DOI: 10.3390/cells10082034
NAFLD-Related Hepatocarcinoma: The Malignant Side of Metabolic Syndrome
Abstract
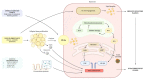
Hepatocellular carcinoma (HCC) is the seventh most common cancer worldwide and the second leading cause of cancer-related mortality. HCC typically arises within a cirrhotic liver, but in about 20% of cases occurs in absence of cirrhosis. Among non-cirrhotic risk factors, non-alcoholic fatty liver disease (NAFLD) currently represents the most important emerging cause of HCC in developed countries. It has been estimated that annual incidence of HCC among patients with non-cirrhotic NAFLD is approximately 0.1-1.3 per 1000 patients/year and ranges from 0.5% to 2.6% among patients with non-alcoholic steatohepatitis (NASH) cirrhosis. However, only a few clinical trials enrolling HCC patients actually distinguished NAFLD/NASH-related cases from other non-cirrhotic causes and therefore evidence is still lacking in this subset of patients. This review aims to describe the biology underpinning NAFLD development, to investigate the main molecular pathways involved in its progression to NASH and HCC and to describe how different pathogenetic mechanisms underlying the onset of HCC can have an impact in clinical practice. We hereby also provide an overview of current HCC treatment options, with a particular focus on the available data on NAFLD-related cases in practice-changing clinical trials.
Keywords: HCC; NAFLD; NASH; hepatocellular carcinoma; insulin resistance; metabolic syndrome.
Conflict of interest statement
F.P. reports grants from AstraZeneca, grants, personal fees and other from Roche, personal fees and other from Eli Lilly, personal fees from Amgen, personal fees from Ipsen, personal fees from MSD, personal fees from Takeda, grants and other from Eisai, others from Novartis and Pfizer, outside the submitted work.
Figures
References
-
- Vogel A., Cervantes A., Chau I., Daniele B., Llovet J.M., Meyer T., Nault J.-C., Neumann U., Ricke J., Sangro B., et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019;30:871–873. doi: 10.1093/annonc/mdy510. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

