High Glycolytic Activity Enhances Stem Cell Reprogramming of Fahd1-KO Mouse Embryonic Fibroblasts
- PMID: 34440809
- PMCID: PMC8392800
- DOI: 10.3390/cells10082040
High Glycolytic Activity Enhances Stem Cell Reprogramming of Fahd1-KO Mouse Embryonic Fibroblasts
Abstract
Mitochondria play a key role in metabolic transitions involved in the reprogramming of somatic cells into induced pluripotent stem cells (iPSCs), but the underlying molecular mechanisms remain largely unexplored. To obtain new insight into the mechanisms of cellular reprogramming, we studied the role of FAH domain-containing protein 1 (FAHD1) in the reprogramming of murine embryonic fibroblasts (MEFs) into iPSCs and their subsequent differentiation into neuronal cells. MEFs from wild type (WT) and Fahd1-knock-out (KO) mice were reprogrammed into iPSCs and characterized for alterations in metabolic parameters and the expression of marker genes indicating mitochondrial biogenesis. Fahd1-KO MEFs showed a higher reprogramming efficiency accompanied by a significant increase in glycolytic activity as compared to WT. We also observed a strong increase of mitochondrial DNA copy number and expression of biogenesis marker genes in Fahd1-KO iPSCs relative to WT. Neuronal differentiation of iPSCs was accompanied by increased expression of mitochondrial biogenesis genes in both WT and Fahd1-KO neurons with higher expression in Fahd1-KO neurons. Together these observations establish a role of FAHD1 as a potential negative regulator of reprogramming and add additional insight into mechanisms by which FAHD1 modulates mitochondrial functions.
Keywords: FAHD1; glycolytic activity; iPSCs; mitochondria; neuronal differentiation; oxidative phosphorylation; reprogramming.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
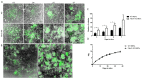
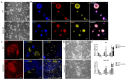


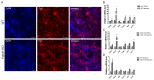
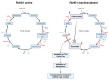
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

