Diversity of Adult Neural Stem and Progenitor Cells in Physiology and Disease
- PMID: 34440814
- PMCID: PMC8392301
- DOI: 10.3390/cells10082045
Diversity of Adult Neural Stem and Progenitor Cells in Physiology and Disease
Abstract
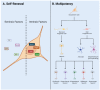
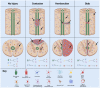
Adult neural stem and progenitor cells (NSPCs) contribute to learning, memory, maintenance of homeostasis, energy metabolism and many other essential processes. They are highly heterogeneous populations that require input from a regionally distinct microenvironment including a mix of neurons, oligodendrocytes, astrocytes, ependymal cells, NG2+ glia, vasculature, cerebrospinal fluid (CSF), and others. The diversity of NSPCs is present in all three major parts of the CNS, i.e., the brain, spinal cord, and retina. Intrinsic and extrinsic signals, e.g., neurotrophic and growth factors, master transcription factors, and mechanical properties of the extracellular matrix (ECM), collectively regulate activities and characteristics of NSPCs: quiescence/survival, proliferation, migration, differentiation, and integration. This review discusses the heterogeneous NSPC populations in the normal physiology and highlights their potentials and roles in injured/diseased states for regenerative medicine.
Keywords: NG2+ cells; central nervous system (CNS); ependymal cells; neural stem and progenitor cells (NSPC); neurodegenerative diseases; regenerative medicine; retina injury; spinal cord injury (SCI); traumatic brain injury (TBI).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

