Secretory Vesicles Are the Principal Means of SARS-CoV-2 Egress
- PMID: 34440816
- PMCID: PMC8393858
- DOI: 10.3390/cells10082047
Secretory Vesicles Are the Principal Means of SARS-CoV-2 Egress
Abstract
The mechanisms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) egress, similar to those of other coronaviruses, remain poorly understood. The virus buds in intracellular compartments and is therefore thought to be released by the biosynthetic secretory pathway. However, several studies have recently challenged this hypothesis. It has been suggested that coronaviruses, including SARS-CoV-2, use lysosomes for egress. In addition, a focused ion-beam scanning electron microscope (FIB/SEM) study suggested the existence of exit tunnels linking cellular compartments rich in viral particles to the extracellular space resembling those observed for the human immunodeficiency (HIV) in macrophages. Here, we analysed serial sections of Vero cells infected with SARS-CoV-2 by transmission electron microscopy (TEM). We found that SARS-CoV-2 was more likely to exit the cell in small secretory vesicles. Virus trafficking within the cells involves small vesicles, with each generally containing a single virus particle. These vesicles then fuse with the plasma membrane to release the virus into the extracellular space. This work sheds new light on the late stages of the SARS-CoV-2 infectious cycle of potential value for guiding the development of new antiviral strategies.
Keywords: COVID-19; SARS-CoV-2; coronavirus; serial sections; transmission electron microscopy; virus egress; virus release.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
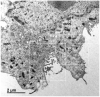

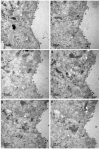
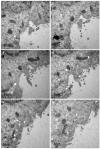



References
-
- World Health Organization WHO Coronavirus Disease (COVID-19) Dashboard. [(accessed on 17 May 2021)]; Available online: https://covid19.who.int/
-
- Snijder E.J., Limpens R.W.A.L., de Wilde A.H., de Jong A.W.M., Zevenhoven-Dobbe J.C., Maier H.J., Faas F.F.G.A., Koster A.J., Bárcena M. A unifying structural and functional model of the coronavirus replication organelle: Tracking down RNA synthesis. PLoS Biol. 2020;18:e3000715. doi: 10.1371/journal.pbio.3000715. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

