Unraveling Human AQP5-PIP Molecular Interaction and Effect on AQP5 Salivary Glands Localization in SS Patients
- PMID: 34440877
- PMCID: PMC8391295
- DOI: 10.3390/cells10082108
Unraveling Human AQP5-PIP Molecular Interaction and Effect on AQP5 Salivary Glands Localization in SS Patients
Abstract
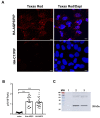
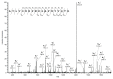
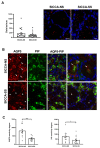
Saliva secretion requires effective translocation of aquaporin 5 (AQP5) water channel to the salivary glands (SGs) acinar apical membrane. Patients with Sjögren's syndrome (SS) display abnormal AQP5 localization within acinar cells from SGs that correlate with sicca manifestation and glands hypofunction. Several proteins such as Prolactin-inducible protein (PIP) may regulate AQP5 trafficking as observed in lacrimal glands from mice. However, the role of the AQP5-PIP complex remains poorly understood. In the present study, we show that PIP interacts with AQP5 in vitro and in mice as well as in human SGs and that PIP misexpression correlates with an altered AQP5 distribution at the acinar apical membrane in PIP knockout mice and SS hMSG. Furthermore, our data show that the protein-protein interaction involves the AQP5 C-terminus and the N-terminal of PIP (one molecule of PIP per AQP5 tetramer). In conclusion, our findings highlight for the first time the role of PIP as a protein controlling AQP5 localization in human salivary glands but extend beyond due to the PIP-AQP5 interaction described in lung and breast cancers.
Keywords: Sjögren’s syndrome; aquaporin-5; prolactin-inducible protein; salivary gland.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Horsefield R., Nordén K., Fellert M., Backmark A., Törnroth-Horsefield S., van Scheltinga A.C.T., Kvassman J., Kjellbom P., Johanson U., Neutze R. High-Resolution x-Ray Structure of Human Aquaporin 5. Proc. Natl. Acad. Sci. USA. 2008;105:13327–13332. doi: 10.1073/pnas.0801466105. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

