Implications of Innate Immunity in Post-Acute Sequelae of Non-Persistent Viral Infections
- PMID: 34440903
- PMCID: PMC8391718
- DOI: 10.3390/cells10082134
Implications of Innate Immunity in Post-Acute Sequelae of Non-Persistent Viral Infections
Abstract
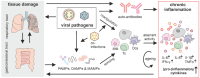
Non-persistent viruses classically cause transient, acute infections triggering immune responses aimed at the elimination of the pathogen. Successful viruses evolved strategies to manipulate and evade these anti-viral defenses. Symptoms during the acute phase are often linked to dysregulated immune responses that disappear once the patient recovers. In some patients, however, symptoms persist or new symptoms emerge beyond the acute phase. Conditions resulting from previous transient infection are termed post-acute sequelae (PAS) and were reported for a wide range of non-persistent viruses such as rota-, influenza- or polioviruses. Here we provide an overview of non-persistent viral pathogens reported to be associated with diverse PAS, among them chronic fatigue, auto-immune disorders, or neurological complications and highlight known mechanistic details. Recently, the emergence of post-acute sequelae of COVID-19 (PASC) or long COVID highlighted the impact of PAS. Notably, PAS of non-persistent infections often resemble symptoms of persistent viral infections, defined by chronic inflammation. Inflammation maintained after the acute phase may be a key driver of PAS of non-persistent viruses. Therefore, we explore current insights into aberrant activation of innate immune signaling pathways in the post-acute phase of non-persistent viruses. Finally, conclusions are drawn and future perspectives for treatment and prevention of PAS are discussed.
Keywords: PASC; acute; chronic; cytokines; inflammation; innate immunity; interferon; long COVID; long hauler syndrome; post-acute; post-acute sequelae; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

