Laboratory Diagnosis of Porphyria
- PMID: 34441276
- PMCID: PMC8391404
- DOI: 10.3390/diagnostics11081343
Laboratory Diagnosis of Porphyria
Abstract
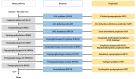
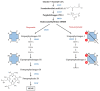
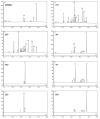
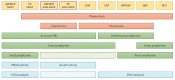
Porphyrias are a group of diseases that are clinically and genetically heterogeneous and originate mostly from inherited dysfunctions of specific enzymes involved in heme biosynthesis. Such dysfunctions result in the excessive production and excretion of the intermediates of the heme biosynthesis pathway in the blood, urine, or feces, and these intermediates are responsible for specific clinical presentations. Porphyrias continue to be underdiagnosed, although laboratory diagnosis based on the measurement of metabolites could be utilized to support clinical suspicion in all symptomatic patients. Moreover, the measurement of enzymatic activities along with a molecular analysis may confirm the diagnosis and are, therefore, crucial for identifying pre-symptomatic carriers. The present review provides an overview of the laboratory assays used most commonly for establishing the diagnosis of porphyria. This would assist the clinicians in prescribing appropriate diagnostic testing and interpreting the testing results.
Keywords: ALA (5-aminolevulinic acid); EPNET (European Porphyria Network); HPLC (high-pressure liquid chromatography); MLPA (multiplex ligation-dependent probe amplification); NGS (next-generation sequencing); PBG (porphobilinogen); diagnosis; porphyria; porphyrins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

