Diagnosis of Mucopolysaccharidoses and Mucolipidosis by Assaying Multiplex Enzymes and Glycosaminoglycans
- PMID: 34441282
- PMCID: PMC8394749
- DOI: 10.3390/diagnostics11081347
Diagnosis of Mucopolysaccharidoses and Mucolipidosis by Assaying Multiplex Enzymes and Glycosaminoglycans
Abstract
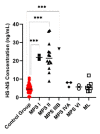
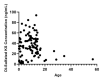
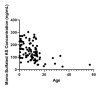
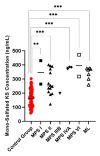
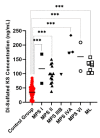
Mucopolysaccharidoses (MPS) and mucolipidosis (ML II/III) are a group of lysosomal storage disorders (LSDs) that occur due to a dysfunction of the lysosomal hydrolases responsible for the catabolism of glycosaminoglycans (GAGs). However, ML is caused by a deficiency of the enzyme uridine-diphosphate N-acetylglucosamine:lysosomal-enzyme-N-acetylglucosamine-1-phosphotransferase (GlcNAc-1-phosphotransferase, EC2.7.8.17), which tags lysosomal enzymes with a mannose 6-phosphate (M6P) marker for transport to the lysosome. A timely diagnosis of MPS and ML can lead to appropriate therapeutic options for patients. To improve the accuracy of diagnosis for MPS and ML in a high-risk population, we propose a combination method based on known biomarkers, enzyme activities, and specific GAGs. We measured five lysosomal enzymes (α-L-iduronidase (MPS I), iduronate-2-sulfatase (MPS II), α-N-acetylglucosaminidase (MPS IIIB), N-acetylglucosamine-6-sulfatase (MPS IVA), and N-acetylglucosamine-4-sulfatase (MPS VI)) and five GAGs (two kinds of heparan sulfate (HS), dermatan sulfate (DS), and two kinds of keratan sulfate (KS)) in dried blood samples (DBS) to diagnose suspected MPS patients by five-plex enzyme and simultaneous five GAGs assays. We used liquid chromatography-tandem mass spectrometry (LC-MS/MS) for both assays. These combined assays were tested for 43 patients with suspected MPS and 103 normal control subjects. We diagnosed two MPS I, thirteen MPS II, one MPS IIIB, three MPS IVA, two MPS VI, and six ML patients with this combined method, where enzymes, GAGs, and clinical manifestations were compatible. The remaining 16 patients were not diagnosed with MPS or ML. The five-plex enzyme assay successfully identified MPS patients from controls. Patients with MPS I, MPS II, and MPS IIIB had significantly elevated HS and DS levels in DBS. Compared to age-matched controls, patients with ML and MPS had significantly elevated mono-sulfated KS and di-sulfated KS levels. The results indicated that the combination method could distinguish these affected patients with MPS or ML from healthy controls. Overall, this study has shown that this combined method is effective and can be implemented in larger populations, including newborn screening.
Keywords: enzyme assay; glycosaminoglycans; mucolipidosis; mucopolysaccharidoses; newborn screening.
Conflict of interest statement
W.J. and T.M. declare competing financial interests: They are employees of Seikagaku Co and provide the technical advices in methods.
Figures


References
-
- Tomatsu S., Mackenzie W.G., Theroux M.C., Mason R.W., Thacker M.M., Shaffer T.H., Montaño A.M., Rowan D., Sly W., Alméciga-Díaz C.J., et al. Current and emerging treatments and surgical interventions for Morquio A Syndrome: A review. Res. Rep. Endocr. Disord. 2012;2012:65–77. doi: 10.2147/RRED.S37278. - DOI - PMC - PubMed
-
- Kubaski F., Yabe H., Suzuki Y., Seto T., Hamazaki T., Mason R.W., Xie L., Onsten T.G.H., Leistner-Segal S., Giugliani R. Hematopoietic stem cell transplantation for patients with mucopolysaccharidosis II. Biol. Blood Marrow Transplant. 2017;23:1795–1803. doi: 10.1016/j.bbmt.2017.06.020. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

