Ciclesonide Inhaler Treatment for Mild-to-Moderate COVID-19: A Randomized, Open-Label, Phase 2 Trial
- PMID: 34441840
- PMCID: PMC8396813
- DOI: 10.3390/jcm10163545
Ciclesonide Inhaler Treatment for Mild-to-Moderate COVID-19: A Randomized, Open-Label, Phase 2 Trial
Abstract
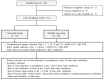
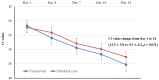
Although some intravenous drugs have been used to treat coronavirus disease 2019 (COVID-19), no effective antiviral agents are currently available in the outpatient setting. We aimed to evaluate the efficacy and adverse events of 14-day ciclesonide treatment vs. standard care for patients with mild-to-moderate COVID-19. A randomized, open-label, multicenter clinical trial of ciclesonide inhalers was conducted in patients with mild-to-moderate COVID-19. Patients were enrolled within 3 days of diagnosis or within 7 days from symptom onset and randomly assigned to receive either ciclesonide (320 µg inhalation twice per day for 14 days) or standard care. The primary endpoint was the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) eradication rate on day 14 from study enrollment. Clinical status was assessed once daily, and serial nasopharyngeal viral load was evaluated by quantitative reverse transcription polymerase chain reaction. There were 35 and 26 patients in the ciclesonide and standard care groups, respectively. The SARS-CoV-2 eradication rate at day 14 was significantly higher in the ciclesonide group (p = 0.021). In multivariate analysis, SARS-CoV-2 negative conversion within 14 days was 12 times more likely in the ciclesonide group (95% confidence interval, 1.187-125.240). Additionally, the clinical failure rate (high-flow nasal oxygen therapy or mechanical ventilation) was significantly lower in the ciclesonide group (p = 0.034). In conclusion, ciclesonide inhalation shortened SARS-CoV-2 viral shedding duration, and it may inhibit the progression to acute respiratory failure in patients with mild-to-moderate COVID-19. Clinical Trial Registration NCT04330586.
Keywords: COVID-19; SARS-CoV-2; antiviral agents; ciclesonide; inhalation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization Coronavirus Disease 2019 (COVID-19): Situation Report. [(accessed on 28 November 2020)]; Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio....
-
- Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., Damiani L.P., Marcadenti A., Kawano-Dourado L., Lisboa T., et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N. Engl. J. Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

