Insights into the Cellular and Molecular Mechanisms That Govern the Fracture-Healing Process: A Narrative Review
- PMID: 34441849
- PMCID: PMC8397080
- DOI: 10.3390/jcm10163554
Insights into the Cellular and Molecular Mechanisms That Govern the Fracture-Healing Process: A Narrative Review
Abstract
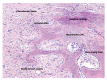
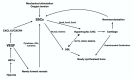
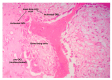
Fracture-healing is a complex multi-stage process that usually progresses flawlessly, resulting in restoration of bone architecture and function. Regrettably, however, a considerable number of fractures fail to heal, resulting in delayed unions or non-unions. This may significantly impact several aspects of a patient's life. Not surprisingly, in the past few years, a substantial amount of research and number of clinical studies have been designed, aiming at shedding light into the cellular and molecular mechanisms that regulate fracture-healing. Herein, we present the current knowledge on the pathobiology of the fracture-healing process. In addition, the role of skeletal cells and the impact of marrow adipose tissue on bone repair is discussed. Unveiling the pathogenetic mechanisms that govern the fracture-healing process may lead to the development of novel, smarter, and more effective therapeutic strategies for the treatment of fractures, especially of those with large bone defects.
Keywords: bone marrow adiposity; bone ossification; bone remodeling; fracture-healing; skeletal stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Nandra R., Grover L., Porter K. Fracture non-union epidemiology and treatment. Trauma. 2016;18:3–11. doi: 10.1177/1460408615591625. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

