A Reperfusion BOLD-MRI Tissue Perfusion Protocol Reliably Differentiate Patients with Peripheral Arterial Occlusive Disease from Healthy Controls
- PMID: 34441939
- PMCID: PMC8397020
- DOI: 10.3390/jcm10163643
A Reperfusion BOLD-MRI Tissue Perfusion Protocol Reliably Differentiate Patients with Peripheral Arterial Occlusive Disease from Healthy Controls
Abstract
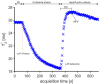
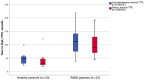
There is no established technique that directly quantifies lower limb tissue perfusion. Blood oxygenation level-dependent magnetic resonance imaging (BOLD-MRI) is an MRI technique that can determine skeletal muscle perfusion. BOLD-MRI relies on magnetic differences of oxygenated and deoxygenated hemoglobin, and regional changes in oxy/deoxyhemoglobin ratio can be recorded by T2* weighted MRI sequences. We aimed to test whether BOLD-MRI can differentiate lower limb tissue perfusion in peripheral arterial occlusive disease (PAOD) patients and healthy controls. Twenty-two PAOD patients and ten healthy elderly volunteers underwent lower limb BOLD-MRI. Reactive hyperemia was provoked by transient cuff compression and images of the gastrocnemius and soleus muscles were continuously acquired at rest, during ischemia and reperfusion. Key BOLD parameters were baseline T2* absolute value and time to T2* peak value after cuff deflation (TTP). Correlations between imaging parameters and ankle-brachial index (ABI) was investigated. The mean TTP was considerably prolonged in PAOD patients compared to healthy controls (m. gastrocnemius: 111 ± 46 versus 48 ± 22 s, p = 0.000253; m. soleus: 100 ± 42 versus 41 ± 30 s, p = 0.000216). Both gastrocnemius and soleus TTP values correlated strongly with ABI (-0.82 and -0.78, p < 0.01). BOLD-MRI during reactive hyperemia differentiated most PAOD patients from healthy controls. TTP was the most decisive parameter and strongly correlated with the ABI.
Keywords: BOLD MRI; atherosclerosis; intermittent claudication; peripheral arterial occlusive disease; tissue perfusion.
Conflict of interest statement
The authors declare no conflict of interest. Carl Sjöberg is employed by the company Antaros Medical. He is involved in the project with scientific interest. We have no financial relationship with the company Antaros itself.
Figures



References
-
- Song P., Rudan D., Zhu Y., Fowkes F.J.I., Rahimi K., Fowkes F.G.R., Rudan I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health. 2019;7:e1020–e1030. doi: 10.1016/S2214-109X(19)30255-4. - DOI - PubMed
-
- Aboyans V., Ricco J.B., Bartelink M.E.L., Bjorck M., Brodmann M., Cohnert T., Collet J.P., Czerny M., De Carlo M., Debus S., et al. Editor’s choice-2017 esc guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the european society for vascular surgery (esvs) Eur. J. Vasc. Endovasc. Surg. 2018;55:305–368. doi: 10.1016/j.ejvs.2017.07.018. - DOI - PubMed
-
- Grozinger G., Pohmann R., Schick F., Grosse U., Syha R., Brechtel K., Rittig K., Martirosian P. Perfusion measurements of the calf in patients with peripheral arterial occlusive disease before and after percutaneous transluminal angioplasty using mr arterial spin labeling. J. Magn. Reson. Imaging. 2014;40:980–987. doi: 10.1002/jmri.24463. - DOI - PubMed
Grants and funding
- Swedish Medical Society (SLS-784061)/Swedish Medical Society (SLS-784061)
- ALFGBG-785741 and ALFGBG-822921/Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG-785741 and ALFGBG-822921)
- 20190194/Swedish Heart-Lung Foundation
- the Sahlgrenska University Hospital Research Fund/the Sahlgrenska University Hospital Research Fund
LinkOut - more resources
Full Text Sources

