Diabetes Mellitus and Heart Failure
- PMID: 34441977
- PMCID: PMC8396967
- DOI: 10.3390/jcm10163682
Diabetes Mellitus and Heart Failure
Abstract
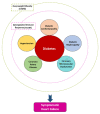
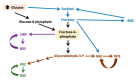
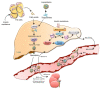
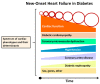
Diabetes mellitus (DM) is a major risk factor for new-onset heart failure (HF) and vice versa. The pathogenesis of new-onset HF in DM is complex and has been largely attributed to the toxic cardiovascular effects of hyperglycemia and relevant metabolic abnormalities (diabetic cardiomyopathy) as well as the frequently coexisting morbidities such as hypertension (HTN), coronary artery disease (CAD), and diabetic nephropathy. In patients with type 1 DM (T1DM), HF develops in the setting of a dysregulated immune response, whereas in most patients with type 2 DM (T2DM), against a background of overweight/obesity. HF prevention in DM is feasible with rigorous treatment of cardiovascular risk factors and selective antidiabetic agents. Conversely, development of new-onset T2DM in HF (cardiogenic DM) is common and has been attributed to an increase in the resistance to insulin, especially in the skeletal muscle, liver, and adipose tissue as well as in diminished insulin secretory response to hyperglycemia by pancreatic β-cells. Cardiogenic DM further deteriorates cardiac dysfunction and adversely affects outcome in HF. Novel lifesaving medications employed in HF management such as sacubitril/valsartan and sodium glucose cotransporter 2 inhibitors (SGLT-2i) have a favorable metabolic profile and lower the incidence of cardiogenic diabetes. Whether mitigation of cardiogenic DM should be a treatment target in HF deserves further investigation.
Keywords: diabetes; diabetic cardiomyopathy; heart failure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Stratton I.M., Adler A.I., Neil H.A., Matthews D.R., Manley S.E., Cull C.A., Hadden D., Turner R.C., Holman R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. Br. Med. J. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. - DOI - PMC - PubMed
-
- Bouthoorn S., Valstar G.B., Gohar A., den Ruijter H.M., Reitsma H.B., Hoes A.W., Rutten F.H. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis. Diab. Vasc. Dis. Res. 2018;15:477–493. doi: 10.1177/1479164118787415. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

