Adverse Events of Percutaneous Microaxial Left Ventricular Assist Devices-A Retrospective, Single-Centre Cohort Study
- PMID: 34442010
- PMCID: PMC8396891
- DOI: 10.3390/jcm10163710
Adverse Events of Percutaneous Microaxial Left Ventricular Assist Devices-A Retrospective, Single-Centre Cohort Study
Abstract
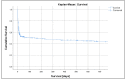
Worldwide, the left ventricular assist device Impella® (Abiomed, Danvers, MA, USA) is increasingly implanted in patients with acute cardiogenic shock or undergoing high-risk cardiac interventions. Despite its long history of use, few studies have assessed its safety and possible complications associated with its use. All patients treated with a left-sided Impella® device at the University Hospital of Basel from 1 January 2011 to 31 December 2019 were enrolled. The primary endpoint was the composite rate of mortality and adverse events (bleeding, acute kidney injury, and limb ischemia). Out of 281 included patients, at least one adverse event was present in 262 patients (93%). Rates of in-hospital, 90-day, and one-year mortality were 48%, 47%, and 50%, respectively. BARC type 3 bleeding (62%) and hemolysis (41.6%) were the most common complications. AKI was observed in 50% of all patients. Renal replacement therapy was required in 97 (35%) of all patients. Limb ischemia occurred in 13% of cases. Bleeding and hemolysis are common Impella®-associated complications. Additionally, we found a high rate of AKI. A careful selection of patients receiving microaxial LV support and defining the indication for its use are essential measures to be taken for the benefits to outweigh potential complications.
Keywords: Impella®; acute heart failure; cardiogenic shock; mechanical circulatory support.
Conflict of interest statement
D.S. received speaker honoraria and educational grants from Abott and Medtronic as well as speaker honoraria from Abiomed and Nycomed.
Figures
References
-
- Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., González-Juanatey J.R., Harjola V.P., Jankowska E.A., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. - DOI - PubMed
-
- Thiele H., Zeymer U. The ESC Textbook of Intensive and Acute Cardiovascular Care Cardiogenic Shock in Patients with Acute Coronary Syndromes. Oxford University Press; Oxford, UK: 2018.
-
- Burzotta F., Trani C., Doshi S.N., Townend J., van Geuns R.J., Hunziker P., Schieffer B., Karatolios K., Moller J.E., Ribichini F.L., et al. Impella ventricular support in clinical practice: Collaborative viewpoint from a European expert user group. Int. J. Cardiol. 2015;201:684–691. doi: 10.1016/j.ijcard.2015.07.065. - DOI - PubMed
-
- Rihal C.S., Naidu S.S., Givertz M.M., Szeto W.Y., Burke J.A., Kapur N.K., Kern M., Garratt K.N., Goldstein J.A., Dimas V., et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care (Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention) J. Card Fail. 2015;21:499–518. doi: 10.1016/j.cardfail.2015.03.002. - DOI - PubMed
-
- Chieffo A., Burzotta F., Pappalardo F., Briguori C., Garbo R., Masiero G., Nicolini E., Ribichini F., Trani C., Alvarez B.C., et al. Clinical expert consensus document on the use of percutaneous left ventricular assist support devices during complex high-risk indicated PCI: Italian Society of Interventional Cardiology Working Group Endorsed by Spanish and Portuguese Interventional Cardiology Societies. Int. J. Cardiol. 2019;293:84–90. doi: 10.1016/j.ijcard.2019.05.065. - DOI - PubMed
LinkOut - more resources
Full Text Sources