Breast Cancer Treatments: Updates and New Challenges
- PMID: 34442452
- PMCID: PMC8399130
- DOI: 10.3390/jpm11080808
Breast Cancer Treatments: Updates and New Challenges
Abstract
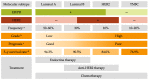
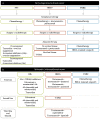
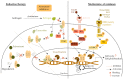
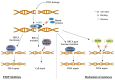
Breast cancer (BC) is the most frequent cancer diagnosed in women worldwide. This heterogeneous disease can be classified into four molecular subtypes (luminal A, luminal B, HER2 and triple-negative breast cancer (TNBC)) according to the expression of the estrogen receptor (ER) and the progesterone receptor (PR), and the overexpression of the human epidermal growth factor receptor 2 (HER2). Current BC treatments target these receptors (endocrine and anti-HER2 therapies) as a personalized treatment. Along with chemotherapy and radiotherapy, these therapies can have severe adverse effects and patients can develop resistance to these agents. Moreover, TNBC do not have standardized treatments. Hence, a deeper understanding of the development of new treatments that are more specific and effective in treating each BC subgroup is key. New approaches have recently emerged such as immunotherapy, conjugated antibodies, and targeting other metabolic pathways. This review summarizes current BC treatments and explores the new treatment strategies from a personalized therapy perspective and the resulting challenges.
Keywords: HER2; TNBC; breast cancer; breast cancer treatment; luminal; molecular subtypes; personalized therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Joshi H., Press M.F. The Breast. Elsevier; Amsterdam, The Netherlands: 2018. [(accessed on 30 May 2021)]. Molecular Oncology of Breast Cancer; pp. 282–307.e5. Available online: https://www.sciencedirect.com/science/article/pii/B9780323359559000222.
-
- Ades F., Zardavas D., Bozovic-Spasojevic I., Pugliano L., Fumagalli D., de Azambuja E., Viale G., Sotiriou C., Piccart M. Luminal B breast cancer: Molecular characterization, clinical management, and future perspectives. J. Clin. Oncol. 2014;32:2794–2803. doi: 10.1200/JCO.2013.54.1870. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

