Towards Biohybrid Lung: Induced Pluripotent Stem Cell Derived Endothelial Cells as Clinically Relevant Cell Source for Biologization
- PMID: 34442603
- PMCID: PMC8401467
- DOI: 10.3390/mi12080981
Towards Biohybrid Lung: Induced Pluripotent Stem Cell Derived Endothelial Cells as Clinically Relevant Cell Source for Biologization
Abstract
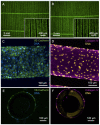
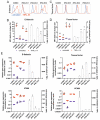
In order to provide an alternative treatment option to lung transplantation for patients with end-stage lung disease, we aim for the development of an implantable biohybrid lung (BHL), based on hollow fiber membrane (HFM) technology used in extracorporeal membrane oxygenators. Complete hemocompatibility of all blood contacting surfaces is crucial for long-lasting BHL durability and can be achieved by their endothelialization. Autologous endothelial cells (ECs) would be the ideal cell source, but their limited proliferation potential excludes them for this purpose. As induced pluripotent stem cell-derived ECs enable the generation of a large number of ECs, we assessed and compared their capacity to form a viable and confluent monolayer on HFM, while indicating physiologic EC-specific anti-thrombogenic and anti-inflammatory properties. ECs were generated from three different human iPSC lines, and seeded onto fibronectin-coated poly-4-methyl-1-pentene (PMP) HFM. Following phenotypical characterization, ECs were analyzed for their thrombogenic and inflammatory behavior with or without TNFα induction, using FACS and qRT-PCR. Complementary, leukocyte- and platelet adhesion assays were carried out. The capacity of the iPSC-ECs to reendothelialize cell-free monolayer areas was assessed in a scratch assay. ECs sourced from umbilical cord blood (hCBECs) were used as control. iPSC-derived ECs formed confluent monolayers on the HFM and showed the typical EC-phenotype by expression of VE-cadherin and collagen-IV. A low protein and gene expression level of E-selectin and tissue factor was detected for all iPSC-ECs and the hCBECs, while a strong upregulation of these markers was noted upon stimulation with TNFα. This was in line with the physiological and strong induction of leukocyte adhesion detected after treatment with TNFα, iPSC-EC and hCBEC monolayers were capable of reducing thrombocyte adhesion and repopulating scratched areas. iPSCs offer the possibility to provide patient-specific ECs in abundant numbers needed to cover all blood contacting surfaces of the BHL with a viable, non-thrombogenic and non-inflammatory monolayer. iPSC-EC clones can differ in terms of their reendothelialization rate, and pro-inflammatory response. However, a less profound inflammatory response may even be advantageous for BHL application. With the proven ability of the seeded iPSC-ECs to reduce thrombocyte adhesion, we expect that thrombotic events that could lead to BHL occlusion can be avoided, and thus, justifies further studies on enabling BHL long-term application.
Keywords: EC activation; biohybrid lung; endothelialization; hemocompatibility; hollow fiber membrane; induced pluripotent stem cells; membrane oxygenator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization (WHO) The Top 10 Causes of Death. [(accessed on 4 July 2021)]; Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
-
- ISHLT Research and Data. [(accessed on 12 June 2021)]; Available online: https://ishlt.org/research-data/registries.
-
- Pham T., Combes A., Rozé H., Chevret S., Mercat A., Roch A., Mourvillier B., Ara-Somohano C., Bastien O., Zogheib E., et al. Extracorporeal Membrane Oxygenation for Pandemic Influenza A(H1N1)–Induced Acute Respiratory Distress Syn-drome. Am. J. Respir. Crit. Care Med. 2013;187:276–285. doi: 10.1164/rccm.201205-0815OC. - DOI - PubMed
-
- Shaefi S., The STOP-COVID Investigators. Brenner S.K., Gupta S., O’Gara B.P., Krajewski M.L., Charytan D.M., Chaudhry S., Mirza S.H., Peev V., et al. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021;47:208–221. doi: 10.1007/s00134-020-06331-9. - DOI - PMC - PubMed
-
- Barbaro R.P., MacLaren G., Boonstra P.S., Iwashyna T.J., Slutsky A.S., Fan E., Bartlett R.H., Tonna J.E., Hyslop R., Fan-ning J.J., et al. Extracorporeal Membrane Oxygenation Support in COVID-19: An International Cohort Study of the Extra-corporeal Life Support Organization Registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

