First Nanoparticles of a Conductor Based on the Organic Donor Molecule BETS: κ-(BETS)2FeCl4
- PMID: 34442966
- PMCID: PMC8398930
- DOI: 10.3390/ma14164444
First Nanoparticles of a Conductor Based on the Organic Donor Molecule BETS: κ-(BETS)2FeCl4
Abstract
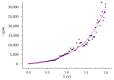
Nanoparticles of the molecular superconductor (BETS)2FeCl4 were obtained by the electrochemical oxidation of BETS in the presence of [(C2H5)4N]FeCl4 and an amphiphilic imine (OATM), acting as a growth controlling agent. When the reaction was carried out with a molar ratio OATM/BETS of 10, roughly spherical nanoparticles exhibiting sizes in the 10-40 nm range were observed. X-ray diffraction patterns evidenced the growth of (BETS)2FeCl4 nanoparticles with the κ-type structure. The current-voltage characteristic recorded on an individual nanoparticle aggregate was fitted with a Shockley diode model. A saturation current of 1216 pA and a threshold voltage of 0.62 V were extracted from this model. This latter value was consistent with roughly half of the energy gap of the semiconducting nano-crystalline aggregate.
Keywords: amphiphilic imine; bis(ethylenedithio)tetraselenafulvalene BETS; electrocrystallization technique; molecular conductors; nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hossick Schott J., Ward M.D. Snapshots of Crystal Growth: Nanoclusters of Organic Conductors on Au(111) Surfaces. J. Am. Chem. Soc. 1994;116:6806–6811. doi: 10.1021/ja00094a039. - DOI
-
- Hillier A.C., Hossick Schott J., Ward M.D. Molecular nanoclusters as precursors to conducting thin films and crystals. Adv. Mater. 1995;7:409–413. doi: 10.1002/adma.19950070416. - DOI
-
- Losilla N.S., Oxtoby N.S., Martinez J., Garcia F., Garcia R., Mas-Torrent M., Veciana J., Rovira C. Sub-50 mm positioning of organic compounds onto silicon oxide patterns fabricated by local oxidation nanolithography. Nanotechnology. 2008;19:45308. doi: 10.1088/0957-4484/19/45/455308. - DOI - PubMed
-
- Jeszka J.K., Tracz A., Wostek D., Boiteux G., Kryszewski M. Preparation of ET2I3 nanocrystals. J. New Mat. Electrochem. Syst. 2001;4:149–153.
-
- Cui G., Xu W., Guo C., Xiao X., Xu H., Zhang D., Jiang L., Zhu D. Conducting Nanopearl Chains Based on the Dmit Salt. J. Phys. Chem. B. 2004;108:13638–13642. doi: 10.1021/jp048195a. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

