Oxidative Aromatization of 4,7-Dihydro-6-nitroazolo[1,5-a]pyrimidines: Synthetic Possibilities and Limitations, Mechanism of Destruction, and the Theoretical and Experimental Substantiation
- PMID: 34443304
- PMCID: PMC8401470
- DOI: 10.3390/molecules26164719
Oxidative Aromatization of 4,7-Dihydro-6-nitroazolo[1,5-a]pyrimidines: Synthetic Possibilities and Limitations, Mechanism of Destruction, and the Theoretical and Experimental Substantiation
Abstract
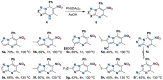
The reaction tolerance of the multicomponent process between 3-aminoazoles, 1-morpholino-2-nitroalkenes, and aldehydes was studied. The main patterns of this reaction have been established. Conditions for the oxidation of 4,7-dihydro-6-nitroazolo[1,5-a]pyrimidines were selected. Previous claims that the 4,7-dihydro-6-nitroazolo[1,5-a]pyrimidines could not be aromatised have now been refuted. Compounds with an electron-donor substituent at position seven undergo decomposition during oxidation. The phenomenon was explained based on experimental data, electro-chemical experiment, and quantum-chemical calculation. The mechanism of oxidative degradation has been proposed.
Keywords: azolo[1,5-a]pyrimidines; multicomponent reactions; nitro compounds; nitro-synthons; oxidation; oxidative destruction; synthesis of heterocycles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Pinheiro S., Pinheiro E.M.C., Muri E.M.F., Pessôa J.C., Cadorini A.M., Greco S.J. Biological Activities of [1,2,4]Triazolo[1,5-a]Pyrimidines and Analogues. Med. Chem. Res. 2020;29:1751–1776. doi: 10.1007/s00044-020-02609-1. - DOI
-
- Fischer G. Advances in Heterocyclic Chemistry. Volume 128. Academic Press Inc.; Cambridge, MA, USA: 2019. Recent Advances in 1,2,4-Triazolo[1,5-a]Pyrimidine Chemistry; pp. 1–101. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

