Solvatomorphism of Moxidectin
- PMID: 34443452
- PMCID: PMC8398095
- DOI: 10.3390/molecules26164869
Solvatomorphism of Moxidectin
Abstract
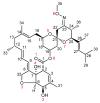
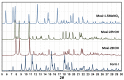
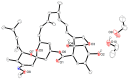
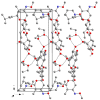
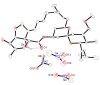
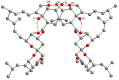
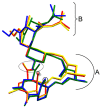
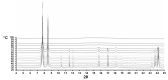
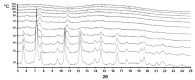
The solvatomorphism of the anthelmintic drug moxidectin is investigated, and a new solvatomorph with nitromethane is reported. Moreover, the hitherto unknown crystal structures of the solvatomorphs with ethanol and 2-propanol are reported and discussed. The thermal characterization of these solvatomorphs through variable-temperature powder X-ray diffraction analysis (VT-PXRD) is also described, providing new insights into the crystallochemistry of this active pharmaceutical ingredient.
Keywords: X-ray diffraction; moxidectin; solvatomorphism; variable-temperature PXRD.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Brittain H.G. Polymorphism in Pharmaceutical Solids. CRC Press; Boca Raton, FL, USA: 2018.
-
- Bernstein J. Polymorphism in Molecular Crystals. Clarendon Press; Oxford, UK: 2007.
-
- Hilfiker R., von Raumer M. Solid Form and Drug Development. John Wiley & Sons Incorporated; Newark, NJ, USA: 2018. Polymorphism in the Pharmaceutical Industry.

