Synthesis of Novel N-Acylhydrazones and Their C-N/N-N Bond Conformational Characterization by NMR Spectroscopy
- PMID: 34443493
- PMCID: PMC8399016
- DOI: 10.3390/molecules26164908
Synthesis of Novel N-Acylhydrazones and Their C-N/N-N Bond Conformational Characterization by NMR Spectroscopy
Abstract
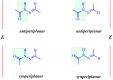
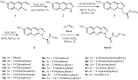
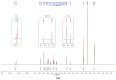
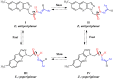
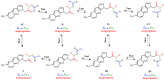
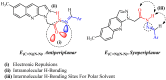
In this article, a synthesis of N'-(benzylidene)-2-(6-methyl-1H-pyrazolo[3,4-b]quinolin-1-yl)acetohydrazides and their structural interpretation by NMR experiments is described in an attempt to explain the duplication of some peaks in their 1H- and 13C-NMR spectra. Twenty new 6-methyl-1H-pyrazolo[3,4-b]quinoline substituted N-acylhydrazones 6(a-t) were synthesized from 2-chloro-6-methylquinoline-3-carbaldehyde (1) in four steps. 2-Chloro-6-methylquinoline-3-carbaldehyde (1) afforded 6-methyl-1H-pyrazolo[3,4-b]quinoline (2), which upon N-alkylation yielded 2-(6-methyl-1H-pyrazolo[3,4-b]quinolin-1-yl)acetate (3). The hydrazinolysis of 3 followed by the condensation of resulting 2-(6-methyl-1H-pyrazolo[3,4-b]quinolin-1-yl)acetohydrazide (4) with aromatic aldehydes gave N-acylhydrazones 6(a-t). Structures of the synthesized compounds were established by readily available techniques such as FT-IR, NMR and mass spectral studies. The stereochemical behavior of 6(a-t) was studied in dimethyl sulfoxide-d6 solvent by means of 1H NMR and 13C NMR techniques at room temperature. NMR spectra revealed the presence of N'-(benzylidene)-2-(6-methyl-1H-pyrazolo[3,4-b]quinolin-1-yl)acetohydrazides as a mixture of two conformers, i.e., E(C=N)(N-N) synperiplanar and E(C=N)(N-N)antiperiplanar at room temperature in DMSO-d6. The ratio of both conformers was also calculated and E(C=N) (N-N) syn-periplanar conformer was established to be in higher percentage in equilibrium with the E(C=N) (N-N)anti-periplanar form.
Keywords: NMR; acylhydrazone; conformer; pyrazolo[3,4-b]quinoline; quinoline; rotamer; stereoisomer.
Conflict of interest statement
The authors declare that they have no significant conflict of interest.
Figures

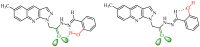
References
-
- Cardoso L.N., Nogueira T.C., Rodrigues F.A., Oliveira A.C.A., dos Santos Luciano M.C., Pessoa C., de Souza M.V. N-acylhydrazones containing thiophene nucleus: A new anticancer class. Med. Chem. Res. 2017;26:1605–1608. doi: 10.1007/s00044-017-1832-y. - DOI
-
- Rozada A.M., Rodrigues F.A., Sampiron E.G., Seixas F.A., Basso E.A., Scodro R.B., Kioshima É.S., Gauze G.F. Novel 4-methoxynaphthalene-N-acylhydrazones as potential for paracoccidioidomycosis and tuberculosis co-infection. Future Microbiol. 2019;14:587–598. doi: 10.2217/fmb-2018-0357. - DOI - PubMed
-
- De Oliveira C.S., Lira B.F., dos Santos Falcão-Silva V., Siqueira-Junior J.P., Barbosa-Filho J.M., Athayde-Filho D., Filgueiras P. Synthesis, molecular properties prediction, and anti-staphylococcal activity of N-acylhydrazones and new 1,3,4-oxadiazole derivatives. Molecules. 2012;17:5095–5107. doi: 10.3390/molecules17055095. - DOI - PMC - PubMed
-
- Nogueira T.C.M., dos Santos Cruz L., Lourenço M.C., de Souza M.V. Design, synthesis and anti-tuberculosis activity of hydrazones and N-acylhydrazones containing vitamin B6 and different heteroaromatic nucleus. Lett. Drug Des. Discov. 2019;16:792–798. doi: 10.2174/1570180815666180627122055. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

