Synthesis and Evaluation of Biological Activities of Bis(spiropyrazolone)cyclopropanes: A Potential Application against Leishmaniasis
- PMID: 34443548
- PMCID: PMC8398714
- DOI: 10.3390/molecules26164960
Synthesis and Evaluation of Biological Activities of Bis(spiropyrazolone)cyclopropanes: A Potential Application against Leishmaniasis
Abstract
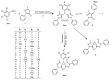
This work focuses on the search and development of drugs that may become new alternatives to the commercial drugs currently available for treatment of leishmaniasis. We have designed and synthesized 12 derivatives of bis(spiropyrazolone)cyclopropanes. We then characterized their potential application in therapeutic use. For this, the in vitro biological activities against three eukaryotic models-S. cerevisiae, five cancer cell lines, and the parasite L. mexicana-were evaluated. In addition, cytotoxicity against non-cancerous mammalian cells has been evaluated and other properties of interest have been characterized, such as genotoxicity, antioxidant properties and, in silico predictive adsorption, distribution, metabolism, and excretion (ADME). The results that we present here represent a first screening, indicating two derivatives of bis(spiropyrazolone)cyclopropanes as good candidates for the treatment of leishmaniasis. They have good specificity against parasites with respect to mammalian cells.
Keywords: ADME; bis(spiropyrazolone)cyclopropanes; drugs; leishmaniasis cytotoxicity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Gomtsyan A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. 2012;48:7–10. doi: 10.1007/s10593-012-0960-z. - DOI
-
- Jamwal A., Javed A., Bhardwaj V. A review on Pyrazole derivatives of pharmacological potential. J. Pharm. Biosci. 2013;3:114–123.
-
- Dewangan D., Kumar T., Alexander A., Nagori K., Tripathi D.K. Pyrazole: Their Chemistry and Pharmacological Potentials: A Review. Curr. Pharma Res. 2011;1:369–377.
-
- Pavlov P.T., Goleneva A.F., Lesnov A.E., Prokhorova T.S. Biological Activity of Some Pyrazolone Derivatives. Pharm. Chem. J. 1998;32:370–372. doi: 10.1007/BF02645994. - DOI
MeSH terms
Substances
Grants and funding
- CEPRA XI-2017-10/Corporación Ecuatoriana para el Desarrollo de la Investigación y la Academia (CEDIA)
- SN/Universidad UTE
- Dirección General de Investigación y Proyectos-DGIP, project #6/Universidad Central del Ecuador
- 9175.0000.0000.377784 and 91750000.0000.375239/SENPLADES
- SN/Académie de Recherche et d´Enseignement Supérieur (ARES)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

