Microwave-Assisted Defibrillation of Microalgae
- PMID: 34443557
- PMCID: PMC8399946
- DOI: 10.3390/molecules26164972
Microwave-Assisted Defibrillation of Microalgae
Abstract
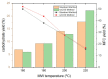
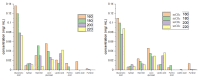
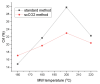
The first production of defibrillated celluloses from microalgal biomass using acid-free, TEMPO-free and bleach-free hydrothermal microwave processing is reported. Two routes were explored: i. direct microwave process of native microalgae ("standard"), and ii. scCO2 pre-treatment followed by microwave processing. ScCO2 was investigated as it is commonly used to extract lipids and generates considerable quantities of spent algal biomass. Defibrillation was evidenced in both cases to afford cellulosic strands, which progressively decreased in their width and length as the microwave processing temperature increased from 160 °C to 220 °C. Lower temperatures revealed aspect ratios similar to microfibrillated cellulose whilst at the highest temperature (220 °C), a mixture of microfibrillated cellulose and nanocrystals were evidenced. XRD studies showed similar patterns to cellulose I but also some unresolved peaks. The crystallinity index (CrI), determined by XRD, increased with increasing microwave processing temperature. The water holding capacity (WHC) of all materials was approximately 4.5 g H2O/g sample. The materials were able to form partially stable hydrogels, but only with those processed above 200 °C and at a concentration of 3 wt% in water. This unique work provides a new set of materials with potential applications in the packaging, food, pharmaceutical and cosmetic industries.
Keywords: defibrillated cellulose; microalgae; microwave processing; zero waste biorefinery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bhatia S.K., Mehariya S., Bhatia R.K., Kumar M., Pugazhendhi A., Awasthi M.K., Atabani A.E., Kumar G., Kim W., Seo S.-O., et al. Wastewater Based Microalgal Biorefinery for Bioenergy Production: Progress and Challenges. Sci. Total Environ. 2021;751:141599. doi: 10.1016/j.scitotenv.2020.141599. - DOI - PubMed
-
- Pavlik D., Zhong Y., Daiek C., Liao W., Morgan R., Clary W., Liu Y. Microalgae Cultivation for Carbon Dioxide Sequestration and Protein Production Using a High-Efficiency Photobioreactor System. Algal Res. 2017;25:413–420. doi: 10.1016/j.algal.2017.06.003. - DOI
-
- Mouahid A., Seengeon K., Martino M., Crampon C., Kramer A., Badens E. Selective Extraction of Neutral Lipids and Pigments from Nannochloropsis Salina and Nannochloropsis Maritima Using Supercritical CO2 Extraction: Effects of Process Parameters and Pre-Treatment. J. Supercrit. Fluids. 2020;165:104934. doi: 10.1016/j.supflu.2020.104934. - DOI
-
- Massa M., Buono S., Langellotti A.L., Martello A., Russo G.L., Troise D.A., Sacchi R., Vitaglione P., Fogliano V. Biochemical Composition and in Vitro Digestibility of Galdieria Sulphuraria Grown on Spent Cherry-Brine Liquid. New Biotechnol. 2019;53:9–15. doi: 10.1016/j.nbt.2019.06.003. - DOI - PubMed
-
- Yadavalli R., Ratnapuram H., Peasari J.R., Reddy C.N., Ashokkumar V., Kuppam C. Simultaneous Production of Astaxanthin and Lipids from Chlorella Sorokiniana in the Presence of Reactive Oxygen Species: A Biorefinery Approach. Biomass Convers. Biorefin. 2021 doi: 10.1007/s13399-021-01276-5. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

