Development of a Novel Nanoarchitecture of the Robust Photosystem I from a Volcanic Microalga Cyanidioschyzon merolae on Single Layer Graphene for Improved Photocurrent Generation
- PMID: 34445103
- PMCID: PMC8395140
- DOI: 10.3390/ijms22168396
Development of a Novel Nanoarchitecture of the Robust Photosystem I from a Volcanic Microalga Cyanidioschyzon merolae on Single Layer Graphene for Improved Photocurrent Generation
Abstract
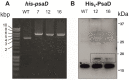
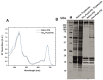
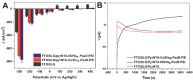
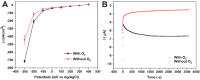
Here, we report the development of a novel photoactive biomolecular nanoarchitecture based on the genetically engineered extremophilic photosystem I (PSI) biophotocatalyst interfaced with a single layer graphene via pyrene-nitrilotriacetic acid self-assembled monolayer (SAM). For the oriented and stable immobilization of the PSI biophotocatalyst, an His6-tag was genetically engineered at the N-terminus of the stromal PsaD subunit of PSI, allowing for the preferential binding of this photoactive complex with its reducing side towards the graphene monolayer. This approach yielded a novel robust and ordered nanoarchitecture designed to generate an efficient direct electron transfer pathway between graphene, the metal redox center in the organic SAM and the photo-oxidized PSI biocatalyst. The nanosystem yielded an overall current output of 16.5 µA·cm-2 for the nickel- and 17.3 µA·cm-2 for the cobalt-based nanoassemblies, and was stable for at least 1 h of continuous standard illumination. The novel green nanosystem described in this work carries the high potential for future applications due to its robustness, highly ordered and simple architecture characterized by the high biophotocatalyst loading as well as simplicity of manufacturing.
Keywords: Cyanidioschyzon merolae; biohybrid nanodevices; biophotovoltaics; direct electron transfer; photosystem I; single layer graphene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
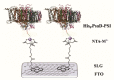



Similar articles
-
Bimodal functionality of highly conductive nanostructured silver film towards improved performance of photosystem I-based graphene photocathode.Bioelectrochemistry. 2025 Feb;161:108825. doi: 10.1016/j.bioelechem.2024.108825. Epub 2024 Sep 19. Bioelectrochemistry. 2025. PMID: 39342775
-
Photocurrent generation from surface assembled photosystem I on alkanethiol modified electrodes.Langmuir. 2013 Feb 19;29(7):2412-9. doi: 10.1021/la304477u. Epub 2013 Feb 4. Langmuir. 2013. PMID: 23379304
-
Photosystem I on graphene as a highly transparent, photoactive electrode.Langmuir. 2013 Apr 2;29(13):4177-80. doi: 10.1021/la305020c. Epub 2013 Mar 22. Langmuir. 2013. PMID: 23506192
-
Growing green electricity: progress and strategies for use of photosystem I for sustainable photovoltaic energy conversion.Biochim Biophys Acta. 2014 Sep;1837(9):1553-66. doi: 10.1016/j.bbabio.2013.12.013. Epub 2014 Jan 3. Biochim Biophys Acta. 2014. PMID: 24388916 Review.
-
Molecular mechanism of photosystem I assembly in oxygenic organisms.Biochim Biophys Acta. 2015 Sep;1847(9):838-48. doi: 10.1016/j.bbabio.2014.12.011. Epub 2015 Jan 9. Biochim Biophys Acta. 2015. PMID: 25582571 Review.
Cited by
-
The coupled photocycle of phenyl-p-benzoquinone and Light-Harvesting Complex II (LHCII) within the biohybrid system.Sci Rep. 2022 Jul 27;12(1):12771. doi: 10.1038/s41598-022-16892-y. Sci Rep. 2022. PMID: 35896789 Free PMC article.
-
Biomolecular and Biohybrid Systems for Solar Energy Conversion.Int J Mol Sci. 2023 Apr 25;24(9):7794. doi: 10.3390/ijms24097794. Int J Mol Sci. 2023. PMID: 37175501 Free PMC article.
-
Improving Photostability of Photosystem I-Based Nanodevice by Plasmonic Interactions with Planar Silver Nanostructures.Int J Mol Sci. 2022 Mar 10;23(6):2976. doi: 10.3390/ijms23062976. Int J Mol Sci. 2022. PMID: 35328397 Free PMC article.
-
Dependence of Protein Immobilization and Photocurrent Generation in PSI-FTO Electrodes on the Electrodeposition Parameters.Int J Mol Sci. 2024 Sep 10;25(18):9772. doi: 10.3390/ijms25189772. Int J Mol Sci. 2024. PMID: 39337260 Free PMC article.
-
Spectral Dependence of the Energy Transfer from Photosynthetic Complexes to Monolayer Graphene.Int J Mol Sci. 2022 Mar 23;23(7):3493. doi: 10.3390/ijms23073493. Int J Mol Sci. 2022. PMID: 35408853 Free PMC article.
References
-
- Solar FAQs.PDF. [(accessed on 9 March 2021)]; Available online: https://old-www.sandia.gov/~jytsao/Solar%20FAQs.pdf.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

